Fractalkine Receptor Deficiency Is Associated with Early Protection but Late Worsening of Outcome following Brain Trauma in Mice - PubMed (original) (raw)
Fractalkine Receptor Deficiency Is Associated with Early Protection but Late Worsening of Outcome following Brain Trauma in Mice
Elisa R Zanier et al. J Neurotrauma. 2016.
Abstract
An impaired ability to regulate microglia activation by fractalkine (CX3CL1) leads to microglia chronic sub-activation. How this condition affects outcome after acute brain injury is still debated, with studies showing contrasting results depending on the timing and the brain pathology. Here, we investigated the early and delayed consequences of fractalkine receptor (CX3CR1) deletion on neurological outcome and on the phenotypical features of the myeloid cells present in the lesions of mice with traumatic brain injury (TBI). Wild type (WT) and CX3CR1(-/-) C57Bl/6 mice were subjected to sham or controlled cortical impact brain injury. Outcome was assessed at 4 days and 5 weeks after TBI by neuroscore, neuronal count, and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining. Compared with WT mice, CX3CR1(-/-) TBI mice showed a significant reduction of sensorimotor deficits and lower cellular damage in the injured cortex 4 days post-TBI. Conversely, at 5 weeks, they showed a worsening of sensorimotor deficits and pericontusional cell death. Microglia (M) and macrophage (μ) activation and polarization were assessed by quantitative immunohistochemistry for CD11b, CD68, Ym1, and inducible nitric oxide synthase (iNOS)-markers of M/μ activation, phagocytosis, M2, and M1 phenotypes, respectively. Morphological analysis revealed a decreased area and perimeter of CD11b(+) cells in CX3CR1(-/-) mice at 4 days post-TBI, whereas, at 5 weeks, both parameters were significantly higher, compared with WT mice. At 4 days, CX3CR1(-/-) mice showed significantly decreased CD68 and iNOS immunoreactivity, while at 5 weeks post-injury, they showed a selective increase of iNOS. Gene expression on CD11b(+) sorted cells revealed an increase of interleukin 10 and insulin-like growth factor 1 (IGF1) at 1 day and a decrease of IGF1 4 days and 5 weeks post-TBI in CX3CR1(-/-), compared with WT mice. These data show an early protection followed by a chronic exacerbation of TBI outcome in the absence of CX3CR1. Thus, longitudinal effects of myeloid cell manipulation at different stages of pathology should be investigated to understand how and when their modulation may offer therapeutic chances.
Keywords: fractalkine receptor; inflammation; macrophages; microglia; traumatic brain injury.
Figures
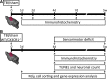
**FIG. 1.
Experimental design. (A) Pattern of microglia/macrophage (M/μ) activation post–traumatic brain injury (TBI) in wild type (WT) mice. Mice were sacrificed 1 day, 2 days, 4 days, 7 days, or 5 weeks after TBI/sham injury for immunohistochemical analysis. (B) Consequences of fractalkine receptor deletion (CX3CR1−/−) on behavioral and histopathological outcome, and on M/μ activation post-TBI. Sensorimotor deficits evaluation and histological analysis were performed in WT and CX3CR1−/− mice 4 days and 5 weeks after TBI/sham injury. Mice were sacrificed at 4 days, 7 days, or 5 weeks for M/μ immunohistochemistry and morphological analysis or at 1 day, 4 days, or 5 weeks for CD11b+ cell sorting by MACS technology and gene expression analysis. Color image is available online at
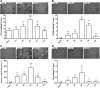
**FIG. 2.
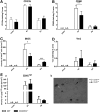
Quantitative immunohistochemical analysis of microglia/macrophage (M/μ) markers in wild type (WT) mice after traumatic brain injury (TBI)/sham injury. Quantification of CD11b (A), CD68 (B), inducible nitric oxide synthase (iNOS) (C), and Ym1 (D) immunostaining in WT mice 1 day, 2 days, 4 days, 7 days, and 5 weeks after TBI/sham injury. Above, representative microphotographs of each marker in sham and TBI mice at the time-point of marker maximal expression and at 5 weeks (bar = 20 μm). Below, quantification of the staining. Data are expressed as mean ± standard deviation of 27 frames/mouse, n = 8. *p < 0.05 vs sham; ^^p < 0.01, ^^^p < 0.001 vs. 4 days for all selected markers, one-way analysis of variance followed by Tukey's post hoc test. n.d., not detected.
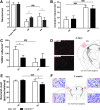
**FIG. 3.
Early and late outcome in wild type (WT) and fractalkine receptor deletion (CX3CR1−/−) mice post–traumatic brain injury (TBI). (A) Functional damage assessed by neuroscore. Before TBI, CX3CR1−/− and WT mice showed a similar neuroscore. After TBI, CX3CR1−/− mice showed a significant reduction of sensorimotor deficits at an early time-point (4 days post-TBI), compared with WT mice. Recovery over time was detected in WT but not in CX3CR1−/− mice, thus leading to a significantly worst performance of CX3CR1−/− mice, compared with WT mice at 5 weeks. (B) Contusion volume. Over time, both WT and CX3CR1−/− mice showed progressive cortical tissue loss with no difference between genotypes. (C, D) DNA damage. WT but not CX3CR1−/− mice showed a decrease in the number of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)+ cells over time post-TBI. Compared with WT mice, CX3CR1−/− mice showed a significant decrease in TUNEL+ cells at 4 days (C) as shown in the representative microphotographs (D). (E, F) Neuronal count. CX3CR1−/− but not WT mice showed a cell loss over time in traumatized cortex. At 5 weeks post-TBI, CX3CR1−/− mice showed a significant lower number of neuronal cells, compared with WT mice (E), as shown in the representative microphotographs (F). Fields for TUNEL evaluation and quantification and for neuronal counts were positioned as depicted in the D and F, respectively. Data are expressed as mean ± standard deviation, n = 11. *p < 0.05, ###p < 0.001, two-way analysis of variance followed by Tukey's post hoc test. Color image is available online at
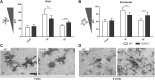
**FIG. 4.
Morphological analysis of microglia/macrophage (M/μ) in wild type (WT) and fractalkine receptor deletion (CX3CR1−/−) mice 4 days and 5 weeks after traumatic brain injury (TBI)/sham injury. CD11b+ cells showed an increase of the area (A) and the perimeter (B) at 4 days after TBI in WT mice, followed by a decrease at 5 weeks post-injury. Compared with WT mice, CX3CR1−/− mice showed a significant reduction of CD11b+ cell area (A) and perimeter (B) at 4 days followed by a significant increase of both parameters 5 weeks after TBI. Representative microphotographs show the typical morphology of M/μ cells in WT and CX3CR1−/− mice at 4 days (C) and 5 weeks (D). Data are reported as mean ± SD, n = 8. **p < 0.01, ***p < 0.001. Two-way analysis of variance followed by Tukey's post hoc test.
**FIG. 5.
Quantitative immunohistochemical analysis of microglia/macrophage (M/μ) markers in wild type (WT) and fractalkine receptor deletion (CX3CR1−/−) mice 4 days and 5 weeks after traumatic brain injury (TBI)/sham injury. (A) No difference between genotypes was detected for CD11b+ immunostaining. (B) Compared with WT mice, CX3CR1−/− mice showed a reduction of lysosomal activity (CD68+ positivity) at 4 days post-TBI. (C) Compared with WT mice, CX3CR1−/− mice showed a significant decrease (4 days) followed by a significant increase (5 weeks) in the density of inducible nitric oxide synthase (iNOS)+ cells. (D, E-a). No differences between genotypes were detected for Ym1 and high expression of CD45 (CD45high) immunostaining. (E-b) Representative microphotograph showing the typical appearance of round-shaped CD45high cells (white arrow) selectively counted. Data are reported as mean ± standard deviation (n = 8). *p < 0.05, ***p < 0.001, two-way analysis of variance followed by Tukey's post hoc test. n.d., not detected.
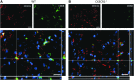
**FIG. 6.
Coexpression of CD11b (red) and CD68 (green) 4 days post–traumatic brain injury in wild type (WT) and fractalkine receptor deletion (CX3CR1−/−) mice. CD68 was greatly increased in CD11b+ cells displaying a hypertrophic morphology in WT mice. CD68 often co-localized with the membrane marker CD11b in WT mice, while it remained mainly located in the cytoplasm in CX3CR1−/− mice. Data are representative of three independent experiments. Bars: 20 μm. Color image is available online at
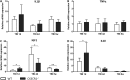
**FIG. 7.
Gene expression analysis of CD11b+ sorted cells in wild type (WT) and fractalkine receptor deletion (CX3CR1−/−) non-injured (sham) mice. (A, B) Compared with WT sham, CX3CR1−/− sham mice had higher interleukin (IL) 1β and tumor necrosis factor (TNF) α messenger RNA expression. (C, D) No differences between genotypes were detected for sham expression of insulin-like growth factor 1 (IGF1) and IL10. Data are expressed as fold of induction, compared with the WT sham group, and are reported as mean ± standard deviation (n = 8). ***p < 0.001, Mann Whitney _t_-test.
**FIG. 8.
Gene expression analysis of CD11b+ sorted cells in wild type (WT) and fractalkine receptor deletion (CX3CR1−/−) mice 1 day, 4 days, and 5 weeks after traumatic brain injury (TBI). Compared with WT mice, CX3CR1−/− mice displayed significantly higher insulin-like growth factor 1 (IGF1) and interleukin (IL) 10 messenger ribonucleic acid (mRNA) expression 1 day post-TBI (C, D). At 4 days and 5 weeks, however, a switch in IGF1 mRNA expression was observed, being IGF1 significantly lower in CX3CR1−/− mice. Data are expressed as fold of induction, compared with the WT sham group, and are reported as mean ± standard deviation (n = 8). *p < 0.05 vs. WT, two-way analysis of variance followed by Tukey's post hoc test.
Similar articles
- Time-dependent effects of CX3CR1 in a mouse model of mild traumatic brain injury.
Febinger HY, Thomasy HE, Pavlova MN, Ringgold KM, Barf PR, George AM, Grillo JN, Bachstetter AD, Garcia JA, Cardona AE, Opp MR, Gemma C. Febinger HY, et al. J Neuroinflammation. 2015 Sep 2;12:154. doi: 10.1186/s12974-015-0386-5. J Neuroinflammation. 2015. PMID: 26329692 Free PMC article. - CX3CR1 deficiency induces an early protective inflammatory environment in ischemic mice.
Fumagalli S, Perego C, Ortolano F, De Simoni MG. Fumagalli S, et al. Glia. 2013 Jun;61(6):827-42. doi: 10.1002/glia.22474. Epub 2013 Feb 26. Glia. 2013. PMID: 23440897 - Anti-inflammatory and immunomodulatory mechanisms of atorvastatin in a murine model of traumatic brain injury.
Xu X, Gao W, Cheng S, Yin D, Li F, Wu Y, Sun D, Zhou S, Wang D, Zhang Y, Jiang R, Zhang J. Xu X, et al. J Neuroinflammation. 2017 Aug 23;14(1):167. doi: 10.1186/s12974-017-0934-2. J Neuroinflammation. 2017. PMID: 28835272 Free PMC article. - Analysis of the Role of CX3CL1 (Fractalkine) and Its Receptor CX3CR1 in Traumatic Brain and Spinal Cord Injury: Insight into Recent Advances in Actions of Neurochemokine Agents.
Poniatowski ŁA, Wojdasiewicz P, Krawczyk M, Szukiewicz D, Gasik R, Kubaszewski Ł, Kurkowska-Jastrzębska I. Poniatowski ŁA, et al. Mol Neurobiol. 2017 Apr;54(3):2167-2188. doi: 10.1007/s12035-016-9787-4. Epub 2016 Mar 1. Mol Neurobiol. 2017. PMID: 26927660 Free PMC article. Review. - Cellular players that shape evolving pathology and neurodegeneration following traumatic brain injury.
Puntambekar SS, Saber M, Lamb BT, Kokiko-Cochran ON. Puntambekar SS, et al. Brain Behav Immun. 2018 Jul;71:9-17. doi: 10.1016/j.bbi.2018.03.033. Epub 2018 Mar 27. Brain Behav Immun. 2018. PMID: 29601944 Review.
Cited by
- Receptors on Microglia.
Augusto-Oliveira M, Tremblay MÈ, Verkhratsky A. Augusto-Oliveira M, et al. Adv Neurobiol. 2024;37:83-121. doi: 10.1007/978-3-031-55529-9_6. Adv Neurobiol. 2024. PMID: 39207688 Review. - L-Norvaline Reverses Cognitive Decline and Synaptic Loss in a Murine Model of Alzheimer's Disease.
Polis B, Srikanth KD, Elliott E, Gil-Henn H, Samson AO. Polis B, et al. Neurotherapeutics. 2018 Oct;15(4):1036-1054. doi: 10.1007/s13311-018-0669-5. Neurotherapeutics. 2018. PMID: 30288668 Free PMC article. - A systemic immune challenge to model hospital-acquired infections independently regulates immune responses after pediatric traumatic brain injury.
Sharma R, Zamani A, Dill LK, Sun M, Chu E, Robinson MJ, O'Brien TJ, Shultz SR, Semple BD. Sharma R, et al. J Neuroinflammation. 2021 Mar 17;18(1):72. doi: 10.1186/s12974-021-02114-1. J Neuroinflammation. 2021. PMID: 33731173 Free PMC article. - Myeloid Pannexin-1 mediates acute leukocyte infiltration and leads to worse outcomes after brain trauma.
Seo JH, Dalal MS, Calderon F, Contreras JE. Seo JH, et al. J Neuroinflammation. 2020 Aug 20;17(1):245. doi: 10.1186/s12974-020-01917-y. J Neuroinflammation. 2020. PMID: 32819386 Free PMC article. - Extrinsic and Intrinsic Mechanisms by Which Mesenchymal Stem Cells Suppress the Immune System.
Coulson-Thomas VJ, Coulson-Thomas YM, Gesteira TF, Kao WW. Coulson-Thomas VJ, et al. Ocul Surf. 2016 Apr;14(2):121-34. doi: 10.1016/j.jtos.2015.11.004. Epub 2016 Jan 12. Ocul Surf. 2016. PMID: 26804815 Free PMC article. Review.
References
- Kumar A. and Loane D.J. (2012). Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain. Behav. Immun. 26, 1191–1201 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous