Structure of the quaternary complex of histone H3-H4 heterodimer with chaperone ASF1 and the replicative helicase subunit MCM2 - PubMed (original) (raw)
Structure of the quaternary complex of histone H3-H4 heterodimer with chaperone ASF1 and the replicative helicase subunit MCM2
Hong Wang et al. Protein Cell. 2015 Sep.
No abstract available
Figures
Figure 1
Structure of the MCM2-ASF1-H3-H4 complex. (A) Schematic diagram showing the truncated fragments of the four proteins. MCM2, ASF1, H3 and H4 are shown in yellow, green, cyan and magenta respectively (color coded the same in all figures). Disordered regions in the structure are shown in gray. An image of the coomassie-stained SDS-PAGE gel of the purified complex showing apparent stoichiometry of the four proteins. MCM2 appears to have an anomalous SDS-PAGE migration profile, as a Mass spectrometric measurement indicates a molecular weight 10,774 Da (data not shown). (B) A ribbon diagram showing the overall structure of the quaternary complex. (C) Interactions between MCM2 and H3-H4 heterodimer. The structure of MCM2 from the ternary MCM2-H3-H4 structure (PDB code: 4UUZ, shown in gray) is superimposed for comparison. Three panels indicate N-terminal, middle and C-terminal binding regions of MCM2. Residues involved in intermolecular interactions are shown in a stick model (carbon, yellow, magenta and cyan; nitrogen, blue; oxygen, red). Dashed lines indicate intermolecular hydrogen bonds. (D) Interactions between ASF1 and histone H3. Superposition of ASF1 and H3 from our quaternary structure and that from the ternary ASF1-H3-H4 structure (PDB code: 2HUE, shown in gray). C-terminal ends of ASF1 in the two structures and α2 MCM2 are highlighted inside the red circle. (E) Interactions between ASF1 and histone H4. The βC strand of H4 in the MCM2-H3-H4 ternary structure and in a human nucleosome structure (shown in gray and magenta, respectively) are aligned with that in the quaternary structure. Histone H2A in the nucleosome structure are shown in red. (F) Direct interactions between MCM2 and ASF1
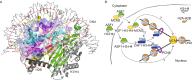
Figure 2
Possible biological functions of the MCM2-ASF1-H3-H4 complex. (A) The binding of MCM2 and ASF1 obstructs the formation of NCP and a histone (H3-H4)2 tetramer. A human nucleosome structure (PDB code: 2CV5, shown in gray) is aligned with the MCM2-ASF1-H3-H4 structure via the H3-H4 heterodimer. Four obstructed regions are enclosed in red circles and numbered according to the order in which they were referenced in the text. (B) A model of possible biological functions of the MCM2-ASF1-H3-H4 quaternary complex in the cytoplasm and in the nucleus. The cytosolic MCM2 may facilitate nuclear import of histones H3 and H4. In the nucleus, ASF1 “hands” the H3-H4 heterodimer to the CAF-1 complex prior to the deposition onto replicated DNA, in the presence or absence of MCM2. The ASF1-H3-H4 complex might also be directly recruited to the replication fork by interaction with the MCM complex
Similar articles
- Structural insight into how the human helicase subunit MCM2 may act as a histone chaperone together with ASF1 at the replication fork.
Richet N, Liu D, Legrand P, Velours C, Corpet A, Gaubert A, Bakail M, Moal-Raisin G, Guerois R, Compper C, Besle A, Guichard B, Almouzni G, Ochsenbein F. Richet N, et al. Nucleic Acids Res. 2015 Feb 18;43(3):1905-17. doi: 10.1093/nar/gkv021. Epub 2015 Jan 23. Nucleic Acids Res. 2015. PMID: 25618846 Free PMC article. - A unique binding mode enables MCM2 to chaperone histones H3-H4 at replication forks.
Huang H, Strømme CB, Saredi G, Hödl M, Strandsby A, González-Aguilera C, Chen S, Groth A, Patel DJ. Huang H, et al. Nat Struct Mol Biol. 2015 Aug;22(8):618-26. doi: 10.1038/nsmb.3055. Epub 2015 Jul 13. Nat Struct Mol Biol. 2015. PMID: 26167883 Free PMC article. - MCM2 binding to histones H3-H4 and ASF1 supports a tetramer-to-dimer model for histone inheritance at the replication fork.
Clément C, Almouzni G. Clément C, et al. Nat Struct Mol Biol. 2015 Aug;22(8):587-9. doi: 10.1038/nsmb.3067. Nat Struct Mol Biol. 2015. PMID: 26243657 No abstract available. - The eukaryotic Mcm2-7 replicative helicase.
Vijayraghavan S, Schwacha A. Vijayraghavan S, et al. Subcell Biochem. 2012;62:113-34. doi: 10.1007/978-94-007-4572-8_7. Subcell Biochem. 2012. PMID: 22918583 Review. - The Role of the MCM2-7 Helicase Subunit MCM2 in Epigenetic Inheritance.
Jia J, Yu C. Jia J, et al. Biology (Basel). 2024 Jul 29;13(8):572. doi: 10.3390/biology13080572. Biology (Basel). 2024. PMID: 39194510 Free PMC article. Review.
Cited by
- The eukaryotic CMG helicase pumpjack and integration into the replisome.
Sun J, Yuan Z, Georgescu R, Li H, O'Donnell M. Sun J, et al. Nucleus. 2016 Apr 25;7(2):146-54. doi: 10.1080/19491034.2016.1174800. Nucleus. 2016. PMID: 27310307 Free PMC article. Review. - Ctf4 organizes sister replisomes and Pol α into a replication factory.
Yuan Z, Georgescu R, Santos RLA, Zhang D, Bai L, Yao NY, Zhao G, O'Donnell ME, Li H. Yuan Z, et al. Elife. 2019 Oct 7;8:e47405. doi: 10.7554/eLife.47405. Elife. 2019. PMID: 31589141 Free PMC article. - ASF1 is required to load histones on the HIRA complex in preparation of paternal chromatin assembly at fertilization.
Horard B, Sapey-Triomphe L, Bonnefoy E, Loppin B. Horard B, et al. Epigenetics Chromatin. 2018 May 11;11(1):19. doi: 10.1186/s13072-018-0189-x. Epigenetics Chromatin. 2018. PMID: 29751847 Free PMC article. - Role of oxidative stress in Retinitis pigmentosa: new involved pathways by an RNA-Seq analysis.
Donato L, Scimone C, Nicocia G, D'Angelo R, Sidoti A. Donato L, et al. Cell Cycle. 2019 Jan;18(1):84-104. doi: 10.1080/15384101.2018.1558873. Epub 2018 Dec 28. Cell Cycle. 2019. PMID: 30569795 Free PMC article. Retracted. - Bacterial and Eukaryotic Replisome Machines.
Yao N, O'Donnell M. Yao N, et al. JSM Biochem Mol Biol. 2016;3(1):1013. Epub 2016 May 30. JSM Biochem Mol Biol. 2016. PMID: 28042596 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous