Profibrotic Role for Interleukin-4 in Cardiac Remodeling and Dysfunction - PubMed (original) (raw)
Profibrotic Role for Interleukin-4 in Cardiac Remodeling and Dysfunction
Hongmei Peng et al. Hypertension. 2015 Sep.
Abstract
Elevated interleukin-4 (IL-4) levels are associated with cardiac fibrosis in hypertension and heart failure in both patients and experimental animals. We hypothesized that chronically elevated IL-4 induces cardiac fibrosis, resulting in a predisposition of the heart to angiotensin II-induced damage. Wild-type Balb/c (WT, high circulating IL-4) and IL-4-deficient Balb/c mice (IL-4(-/-)) were used. WT mice exhibited cardiac fibrosis (evidenced by an increase in expression of procollagen genes/interstitial collagen fraction), enlarged left ventricle chamber, and declined cardiac function associated with a greater number of mast cells and macrophages in the heart compared with IL-4(-/-). In contrast, IL-4(-/-) mice had normal cardiac architecture/function while showing a 57.9% reduction in heart interstitial collagen compared with WT, despite elevated proinflammatory cytokines in heart tissue. In response to angiotensin II administration, IL-4(-/-) had reduced interstitial myocardial fibrosis and were protected from developing dilated cardiomyopathy, which was seen in WT mice. This was associated with increased macrophage infiltration into the hearts of WT mice, despite a similar degree of hypertension and increased cardiac transforming growth factor-β1 in both groups. In vitro data demonstrated that IL-4 upregulates procollagen genes and stimulates collagen production in mouse cardiac fibroblasts. This process is mediated by signal transducer and activator of transcription 6 signaling pathway via IL-4 receptor alpha. This study not only establishes a causal relationship between IL-4 and cardiac fibrosis/dysfunction, but also reveals a critical role for IL-4 in angiotensin II-induced cardiac damage. IL-4 could serve as an additional target for the treatment of cardiac fibrosis.
Keywords: angiotensin II; balb/c mice; fibrosis; heart; interleukin-4.
© 2015 American Heart Association, Inc.
Figures
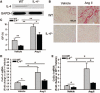
Figure 1
Systolic blood pressure (SBP) and cardiac remodeling in wild-type (WT) and interleukin-4 (IL-4)−/− mice at the steady-state condition and angiotensin (Ang) II–induced hypertension. A, SBP data. Results represent mean±SEM. #P<0.005 vs WT+vehicle; *P<0.005 vs IL-4−/−+vehicle, a Student's t test with a Hochberg correction for multiple testing. Cardiac hypertrophy/remodeling and function assessed by left ventricle weight (LVW) to tibia length (TL) ratio (B) or echocardiography as the sum of diastolic posterior wall thickness (PWT; C), left ventricular diastolic dimension (LVDd; D), ejection fraction (E), and shortening fraction (F) after 8 weeks of Ang II treatment in WT and IL-4−/− mice. The bars represent mean±SEM. *P<0.05, **P<0.005, a 2-sample 2-sided Wilcoxon test with a Hochberg correction for multiple testing, n=6 to 14 per group.
Figure 2
Cardiac fibrotic remodeling in the hearts of wild-type (WT) and interleukin-4 (IL-4)−/− mice. A, Left ventricle lysates were analyzed for IL-4 by immunoblotting with an antibody against mouse IL-4. Representative images of interstitial fibrillar collagen (red) in the hearts of mice treated with either vehicle or angiotensin (Ang) II for 8 weeks (B) and quantification of interstitial collagen fraction (ICF) of the study animals (C). Procollagen type-I alpha 1 (Col1α1; D) mRNA and procollagen type-III alpha 1 (Col3α1; E) mRNA in the myocardium of mice treated with either vehicle or Ang II for 8 weeks. The bars represent mean±SEM, *P<0.05 and **P<0.005, a 2-sample 2-sided Wilcoxon test with a Hochberg correction for multiple testing, n=6 to 14 per group.
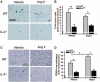
Figure 3
Mast cells and CD68+ macrophages in the hearts of wild-type (WT) and interleukin-4 (IL-4)−/− mice. Representative images of mast cells (purple; A) and CD68+ macrophages (red-brown; C) in the myocardium of mice treated with either vehicle or angiotensin (Ang) II for 8 weeks and quantification of the numbers of mast cells (B) and CD68+ macrophages (D). The bars represent mean±SEM, *P<0.05, **P<0.005, a 2-sample 2-sided Wilcoxon test with a Hochberg correction for multiple testing, n=5 to 9 per group.
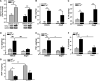
Figure 4
Cytokine and monocyte chemoattractant protein-1 (MCP-1) expression in the hearts of wild-type (WT) and interleukin (IL)-4−/− mice. Left ventricle lysates from WT and IL-4−/− mice treated with either vehicle or angiotensin (Ang) II were processed by Western blot for transforming growth factor-β1 (TGF-β1; A) by a cytokine bead array for interferon gamma (IFNγ), IL-10, IL-2, IL-1β, and tumor necrosis factor-α (TNFα; B-F) and by ELISA for MCP-1 (G). The bars represent mean±SEM, *P<0.05, **P<0.005, a 2-sample 2-sided Wilcoxon test with a Hochberg correction for multiple testing, n=4 to 5 per group.
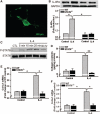
Figure 5
Effects of interleukin-4 (IL-4) on cultured mouse cardiac fibroblasts. A, Representative immunofluorescent image of cell-surface IL-4 receptor alpha (IL-4Rα) on mouse cardiac fibroblasts (green). Primary cardiac fibroblasts were prepared from wild-type (WT) or signal transducer, and activator of transcription 6 (STAT6)−/− mice, IL-4Rα expression (B), and IL-4–induced phosphorylated STAT6 (P-STAT6; C) were analyzed by Western blot. A and C, Results from 3 independent experiments. D, Collagen contents in conditioned media of cardiac fibroblasts treated with IL-4 (10 ng/mL) for 48 hours. mRNA abundance of procollagen type-I α1 (Col1α1; E) and procollagen type-III α1 (Col3α1; F) after cells were incubated with IL-4 (10 ng/mL) for 6 hours. The bars represent mean±SEM, *P<0.05, **P<0.005, a 2-sample 2-sided Wilcoxon test with a Hochberg correction for multiple testing, n=4 per group.
Similar articles
- C-reactive protein promotes cardiac fibrosis and inflammation in angiotensin II-induced hypertensive cardiac disease.
Zhang R, Zhang YY, Huang XR, Wu Y, Chung AC, Wu EX, Szalai AJ, Wong BC, Lau CP, Lan HY. Zhang R, et al. Hypertension. 2010 Apr;55(4):953-60. doi: 10.1161/HYPERTENSIONAHA.109.140608. Epub 2010 Feb 15. Hypertension. 2010. PMID: 20157054 - Tumor Necrosis Factor - Alpha Is Essential for Angiotensin II-Induced Ventricular Remodeling: Role for Oxidative Stress.
Sriramula S, Francis J. Sriramula S, et al. PLoS One. 2015 Sep 17;10(9):e0138372. doi: 10.1371/journal.pone.0138372. eCollection 2015. PLoS One. 2015. PMID: 26378790 Free PMC article. - Eplerenone attenuates myocardial fibrosis in the angiotensin II-induced hypertensive mouse: involvement of tenascin-C induced by aldosterone-mediated inflammation.
Nishioka T, Suzuki M, Onishi K, Takakura N, Inada H, Yoshida T, Hiroe M, Imanaka-Yoshida K. Nishioka T, et al. J Cardiovasc Pharmacol. 2007 May;49(5):261-8. doi: 10.1097/FJC.0b013e318033dfd4. J Cardiovasc Pharmacol. 2007. PMID: 17513943 - Large blood pressure variability and hypertensive cardiac remodeling--role of cardiac inflammation.
Kai H, Kudo H, Takayama N, Yasuoka S, Kajimoto H, Imaizumi T. Kai H, et al. Circ J. 2009 Dec;73(12):2198-203. doi: 10.1253/circj.cj-09-0741. Epub 2009 Oct 29. Circ J. 2009. PMID: 19875896 Review. - Angiotensin II induces inflammation leading to cardiac remodeling.
Jia L, Li Y, Xiao C, Du J. Jia L, et al. Front Biosci (Landmark Ed). 2012 Jan 1;17(1):221-31. doi: 10.2741/3923. Front Biosci (Landmark Ed). 2012. PMID: 22201740 Review.
Cited by
- Fibroblasts orchestrate cellular crosstalk in the heart through the ECM.
Bowers SLK, Meng Q, Molkentin JD. Bowers SLK, et al. Nat Cardiovasc Res. 2022 Apr;1(4):312-321. doi: 10.1038/s44161-022-00043-7. Epub 2022 Apr 13. Nat Cardiovasc Res. 2022. PMID: 38765890 Free PMC article. - Indoxyl sulphate-initiated activation of cardiac fibroblasts is modulated by aryl hydrocarbon receptor and nuclear factor-erythroid-2-related factor 2.
Barisione C, Verzola D, Garibaldi S, Altieri P, Furfaro AL, Nitti M, Pratesi G, Palombo D, Ameri P. Barisione C, et al. J Cell Mol Med. 2024 Apr;28(7):e18192. doi: 10.1111/jcmm.18192. J Cell Mol Med. 2024. PMID: 38506079 Free PMC article. - Whole blood transcriptomics reveals granulocyte colony-stimulating factor as a mediator of cardiopulmonary bypass-induced systemic inflammatory response syndrome.
Martin KR, Gamell C, Tai TY, Bonelli R, Hansen J, Tatoulis J, Alhamdoosh M, Wilson N, Wicks I. Martin KR, et al. Clin Transl Immunology. 2024 Feb 19;13(2):e1490. doi: 10.1002/cti2.1490. eCollection 2024. Clin Transl Immunology. 2024. PMID: 38375330 Free PMC article. - Emerging Perspectives on the Set of Conditions That Lead to the Emergence of Metabolic Syndrome.
Tarcău BM, Vicaș LG, Filip L, Maghiar F, Șandor M, Pallag A, Jurca T, Mureșan ME, Marian E. Tarcău BM, et al. J Pers Med. 2023 Dec 26;14(1):32. doi: 10.3390/jpm14010032. J Pers Med. 2023. PMID: 38248733 Free PMC article. Review. - Pro-Fibrotic Role of Interleukin-4 in Influencing Idiopathic Epiretinal Membrane in Cataract Patients: Analysis From Clinical-Experimental Approaches.
Song P, Li P, Huang Z, Yuan Y, Wei M, Wang C, Zhang G, Ji M, Guan H. Song P, et al. Transl Vis Sci Technol. 2023 Nov 1;12(11):23. doi: 10.1167/tvst.12.11.23. Transl Vis Sci Technol. 2023. PMID: 37982769 Free PMC article.
References
- Vasan RS, Benjamin EJ. Diastolic heart failure–no time to relax. N Engl J Med. 2001;344:56–59. doi: 10.1056/NEJM200101043440111. - PubMed
- Roselló-Lletí E, Rivera M, Bertomeu V, Cortés R, Jordán A, González-Molina A. [Interleukin-4 and cardiac fibrosis in patients with heart failure]. Rev Esp Cardiol. 2007;60:777–780. - PubMed
- Catapano G, Pedone C, Nunziata E, Zizzo A, Passantino A, Incalzi RA. Nutrient intake and serum cytokine pattern in elderly people with heart failure. Eur J Heart Fail. 2008;10:428–434. doi: 10.1016/j.ejheart.2008.02.016. - PubMed
- Levick SP, McLarty JL, Murray DB, Freeman RM, Carver WE, Brower GL. Cardiac mast cells mediate left ventricular fibrosis in the hyper-tensive rat heart. Hypertension. 2009;53:1041–1047. doi: 10.1161/HYPERTENSIONAHA.108.123158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials