Keratinocytes can modulate and directly initiate nociceptive responses - PubMed (original) (raw)
Keratinocytes can modulate and directly initiate nociceptive responses
Kyle M Baumbauer et al. Elife. 2015.
Abstract
How thermal, mechanical and chemical stimuli applied to the skin are transduced into signals transmitted by peripheral neurons to the CNS is an area of intense study. Several studies indicate that transduction mechanisms are intrinsic to cutaneous neurons and that epidermal keratinocytes only modulate this transduction. Using mice expressing channelrhodopsin (ChR2) in keratinocytes we show that blue light activation of the epidermis alone can produce action potentials (APs) in multiple types of cutaneous sensory neurons including SA1, A-HTMR, CM, CH, CMC, CMH and CMHC fiber types. In loss of function studies, yellow light stimulation of keratinocytes that express halorhodopsin reduced AP generation in response to naturalistic stimuli. These findings support the idea that intrinsic sensory transduction mechanisms in epidermal keratinocytes can directly elicit AP firing in nociceptive as well as tactile sensory afferents and suggest a significantly expanded role for the epidermis in sensory processing.
Keywords: epidermal; keratinocyte; mouse; neuroscience; nociceptor; optogenetics; pain; sensory neuron.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
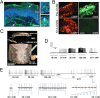
Figure 1.. Light stimulates various types of cutaneous afferents in Prph-ChR2 transgenic mice.
(A). ChR2-YFP expression in unmyelinated and myelinated (lanceolate endings of hair shaft, panels on right) fibers of Prph-ChR2 mouse skin. Arrows indicate nerve fibers in dermis and epidermis (Epi); DAPI (blue) labeling demarcates keratinocytes. (B). ChR2 is expressed in DRG neurons of Prph-ChR2 but not KRT-ChR2 mice. CGRP labels peptidergic neurons. (C). Ex vivo preparation used for functional characterization of cutaneous afferents in response to mechanical, heat and laser stimulation. (D). Response of a Prph-ChR2 Aδ-HTMR to mechanical and blue laser stimulation. (E). Recordings from a CMHC nociceptor from a Prph-ChR2 mouse in response to mechanical, thermal and light stimulation. Calibration bars in (A) = 250 µm, (B) = 100 µm, (E) = 60 mV/1 s, top trace; 40 mV/1 s, bottom trace. DOI:
http://dx.doi.org/10.7554/eLife.09674.003
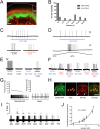
Figure 2.. Blue light stimulates multiple subtypes of cutaneous afferents in KRT-ChR2 transgenic mice.
(A). ChR2-YFP expression in keratinocytes of glabrous skin of KRT-ChR2 mouse. PGP9.5-positive nerve fibers (red) are in dermis and epidermis (arrows). (B). Plot of behavioral responses to blue laser across time intervals for Prph-ChR2 and KRT-ChR2 mice. All Prph-Cre mice showed an immediate response (within 5 s of stimulation). All KRT-ChR2 mice also responded at least once in 10 trials and with variable latencies (see Table 1). (C). Example showing activation of a CMH fiber type in response to blue laser applied to KRT-ChR2 skin in the ex vivo preparation. Responses of this fiber to mechanical and heat stimuli are shown below laser response. (D). Example of a train of action potentials elicited in a CH fiber type in response to laser activation of the KRT-ChR2 skin. Responses of this fiber to heat stimuli are shown below laser response. (E). In this KRT-ChR2 Aβ HTMR afferent laser stimulation does not produce firing when presented alone, but does in combination with subthreshold (5 mN) mechanical stimulation. (F). Light directly activates this KRT-ChR2 CMHC fiber and summates with noxious heat stimulation. (G). SA1 Aβ-low threshold mechanoreceptor responds to mechanical and laser stimulation. (H). SA1s terminate on ChR2-YFP (green) positive Merkel cells co-labeled with anti-K20 (orange). Anti-NFH (red) labels SA1 fiber. Calibration bars in (A) and (H) = 100 µm. (I). Light-evoked responses from a SA-1 fiber at varying intensities (1–40 mW) with instantaneous frequency depicted. Pulses were 5 s in duration with 30 s between pulses. (J). Normalized mean firing rate vs light intensity plotted on a log-intensity scale. Data from 8 afferents are averaged from ascending and descending steps of light intensity, and were fit with a Boltzman sigmoidal function (R2 = 0.98). DOI:
http://dx.doi.org/10.7554/eLife.09674.005
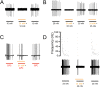
Figure 3.. Light elicits current activation in cultured keratinocytes.
(A). Fluorescent ChR2-YFP protein in plasma membrane of keratinocytes cultured from skin of KRT-ChR2 mice. (B). IR-DIC images of patch pipette on single keratinocyte that was recorded from and then filled with Alexa 555 dye. (C). Representative trace illustrates typical current evoked by blue light stimulation of KRT-ChR2. Yellow light stimulation of KRT-NpHR keratinocytes also produced a change in voltage properties of the cell. Control KRT-Cre keratinocytes that were isolated in parallel showed no response to light (not shown). Bar in A is 40 µM. DOI:
http://dx.doi.org/10.7554/eLife.09674.008
Figure 4.. Yellow light inhibits AP firing in multiple subtypes of cutaneous afferents in KRT-NpHR mice.
(A). Yellow light decreases AP firing in response to mechanical stimulation in this Aδ-HTMR afferent. (B). In this CMH-fiber the response to mechanical stimulation is decreased with the initial yellow laser stimulation; a smaller decrease in AP firing occurred with a second laser presentation. (C). This CMH-fiber showed decreased firing in response to heat in the presence of yellow laser stimulation. (D). Responses of a SA1 fiber to mechanical stimulation are significantly reduced by activation of NpHR in epidermal keratinocytes (which are likely Merkel cells). Laser stimuli (orange bars) occurred 1 s prior to mechanical (black bar) or heat (red bar) stimuli. Duration of each stimulus was either 5 s (mechanical and heat) or 6 s (laser). DOI:
http://dx.doi.org/10.7554/eLife.09674.009
Similar articles
- Optogenetic Activation of Colon Epithelium of the Mouse Produces High-Frequency Bursting in Extrinsic Colon Afferents and Engages Visceromotor Responses.
Makadia PA, Najjar SA, Saloman JL, Adelman P, Feng B, Margiotta JF, Albers KM, Davis BM. Makadia PA, et al. J Neurosci. 2018 Jun 20;38(25):5788-5798. doi: 10.1523/JNEUROSCI.0837-18.2018. Epub 2018 May 22. J Neurosci. 2018. PMID: 29789376 Free PMC article. - Keratinocyte PIEZO1 modulates cutaneous mechanosensation.
Mikesell AR, Isaeva O, Moehring F, Sadler KE, Menzel AD, Stucky CL. Mikesell AR, et al. Elife. 2022 Sep 2;11:e65987. doi: 10.7554/eLife.65987. Elife. 2022. PMID: 36053009 Free PMC article. - What about physical contacts between epidermal keratinocytes and sensory neurons?
Talagas M, Lebonvallet N, Leschiera R, Marcorelles P, Misery L. Talagas M, et al. Exp Dermatol. 2018 Jan;27(1):9-13. doi: 10.1111/exd.13411. Epub 2017 Oct 19. Exp Dermatol. 2018. PMID: 28767170 Review. - Keratinocytes mediate innocuous and noxious touch via ATP-P2X4 signaling.
Moehring F, Cowie AM, Menzel AD, Weyer AD, Grzybowski M, Arzua T, Geurts AM, Palygin O, Stucky CL. Moehring F, et al. Elife. 2018 Jan 16;7:e31684. doi: 10.7554/eLife.31684. Elife. 2018. PMID: 29336303 Free PMC article.
Cited by
- Neuroendocrine signaling in the skin with a special focus on the epidermal neuropeptides.
Slominski AT, Slominski RM, Raman C, Chen JY, Athar M, Elmets C. Slominski AT, et al. Am J Physiol Cell Physiol. 2022 Dec 1;323(6):C1757-C1776. doi: 10.1152/ajpcell.00147.2022. Epub 2022 Nov 1. Am J Physiol Cell Physiol. 2022. PMID: 36317800 Free PMC article. Review. - Neurological Disturbances of Ciguatera Poisoning: Clinical Features and Pathophysiological Basis.
L'Herondelle K, Talagas M, Mignen O, Misery L, Le Garrec R. L'Herondelle K, et al. Cells. 2020 Oct 14;9(10):2291. doi: 10.3390/cells9102291. Cells. 2020. PMID: 33066435 Free PMC article. Review. - Electrical aspects of skin as a pathway to engineering skin devices.
Abe Y, Nishizawa M. Abe Y, et al. APL Bioeng. 2021 Nov 18;5(4):041509. doi: 10.1063/5.0064529. eCollection 2021 Dec. APL Bioeng. 2021. PMID: 34849444 Free PMC article. Review. - Control of mechanical pain hypersensitivity in mice through ligand-targeted photoablation of TrkB-positive sensory neurons.
Dhandapani R, Arokiaraj CM, Taberner FJ, Pacifico P, Raja S, Nocchi L, Portulano C, Franciosa F, Maffei M, Hussain AF, de Castro Reis F, Reymond L, Perlas E, Garcovich S, Barth S, Johnsson K, Lechner SG, Heppenstall PA. Dhandapani R, et al. Nat Commun. 2018 Apr 24;9(1):1640. doi: 10.1038/s41467-018-04049-3. Nat Commun. 2018. PMID: 29691410 Free PMC article. - Transient Receptor Potential Vanilloid 4 Ion Channel Functions as a Pruriceptor in Epidermal Keratinocytes to Evoke Histaminergic Itch.
Chen Y, Fang Q, Wang Z, Zhang JY, MacLeod AS, Hall RP, Liedtke WB. Chen Y, et al. J Biol Chem. 2016 May 6;291(19):10252-62. doi: 10.1074/jbc.M116.716464. Epub 2016 Mar 9. J Biol Chem. 2016. PMID: 26961876 Free PMC article.
References
- Baumbauer K, DeBerry JJ, Adelman P, Miller R, Davis BM, Albers KM, Koerber HR. Optical stimulation of keratinocytes activates cutaneous nociceptors. Washington, D.C.: Society for Neuroscience; 2014.
Publication types
MeSH terms
Grants and funding
- UL1 TR000005/TR/NCATS NIH HHS/United States
- AR063772/AR/NIAMS NIH HHS/United States
- T32 NS073548/NS/NINDS NIH HHS/United States
- NS023725/NS/NINDS NIH HHS/United States
- R56 NS023725/NS/NINDS NIH HHS/United States
- NS033730/NS/NINDS NIH HHS/United States
- R01 NS050758/NS/NINDS NIH HHS/United States
- T32 DK063922/DK/NIDDK NIH HHS/United States
- R01 NS023725/NS/NINDS NIH HHS/United States
- R03 NS075760/NS/NINDS NIH HHS/United States
- NS075760/NS/NINDS NIH HHS/United States
- R01 AR063772/AR/NIAMS NIH HHS/United States
- K01 DK101681/DK/NIDDK NIH HHS/United States
- R01 AR069951/AR/NIAMS NIH HHS/United States
- R21 AR066371/AR/NIAMS NIH HHS/United States
- R01 NS033730/NS/NINDS NIH HHS/United States
- NS050758/NS/NINDS NIH HHS/United States
- AR066371/AR/NIAMS NIH HHS/United States
- DK063922/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous