Sympathetic fibre sprouting in the skin contributes to pain-related behaviour in spared nerve injury and cuff models of neuropathic pain - PubMed (original) (raw)
Sympathetic fibre sprouting in the skin contributes to pain-related behaviour in spared nerve injury and cuff models of neuropathic pain
Francisney P Nascimento et al. Mol Pain. 2015.
Abstract
Background: Cuff and spared nerve injury (SNI) in the sciatic territory are widely used to model neuropathic pain. Because nociceptive information is first detected in skin, it is important to understand how alterations in peripheral innervation contribute to pain in each model. Over 16 weeks in male rats, changes in sensory and autonomic innervation of the skin were described after cuff and SNI using immunohistochemistry to label myelinated (neurofilament 200 positive-NF200+) and peptidergic (calcitonin gene-related peptide positive-CGRP+) primary afferents and sympathetic fibres (dopamine β-hydroxylase positive-DBH+)
Results: Cuff and SNI caused an early loss and later reinnervation of NF200 and CGRP fibres in the plantar hind paw skin. In both models, DBH+ fibres sprouted into the upper dermis of the plantar skin 4 and 6 weeks after injury. Despite these similarities, behavioural pain measures were significantly different in each model. Sympathectomy using guanethidine significantly alleviated mechanical allodynia 6 weeks after cuff, when peak sympathetic sprouting was observed, having no effect at 2 weeks, when fibres were absent. In SNI animals, mechanical allodynia in the lateral paw was significantly improved by guanethidine at 2 and 6 weeks, and the development of cold hyperalgesia, which roughly paralleled the appearance of ectopic sympathetic fibres, was alleviated by guanethidine at 6 weeks. Sympathetic fibres did not sprout into the dorsal root ganglia at 2 or 6 weeks, indicating their unimportance to pain behaviour in these two models.
Conclusions: Alterations in sympathetic innervation in the skin represents an important mechanism that contributes to pain in cuff and SNI models of neuropathic pain.
Figures
Fig. 1
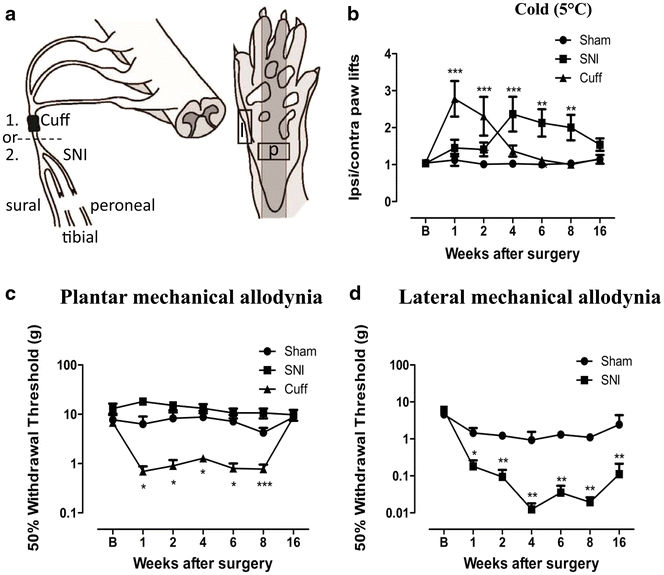
Cuff and SNI rats develop mechanical allodynia and cold hyperalgesia. a Illustration of the cuff and SNI models of neuropathic pain and the regions of the paw targeted for behaviour and innervation changes. Animals received either a cuff or a SNI surgery. (1) The cuff model involves the application of a polyethylene cuff around the sciatic nerve before it branches. (2) SNI involves the transection of the tibial and peroneal branches of the sciatic nerve, leaving the sural nerve intact. Squares indicate the region of the paw where behaviour was tested and innervation changes measured. Only the plantar paw (p) was tested in cuff animals, whereas the lateral paw (l), innervated by the spared sural nerve, was also targeted in SNI animals. b Responses to cold plate set to 5 °C in cuff, SNI and sham rats. Values represent the paw lift ratio between ipsilateral/contralateral paws. c 50 % withdrawal threshold to von Frey fibres in the plantar paw of cuff, SNI and sham rats. d 50 % withdrawal threshold to von Frey fibres in the lateral paw of SNI and sham rats. Each point represents the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 compared to sham by two way ANOVA with Bonferroni post hoc test (n = 8 per group)
Fig. 2
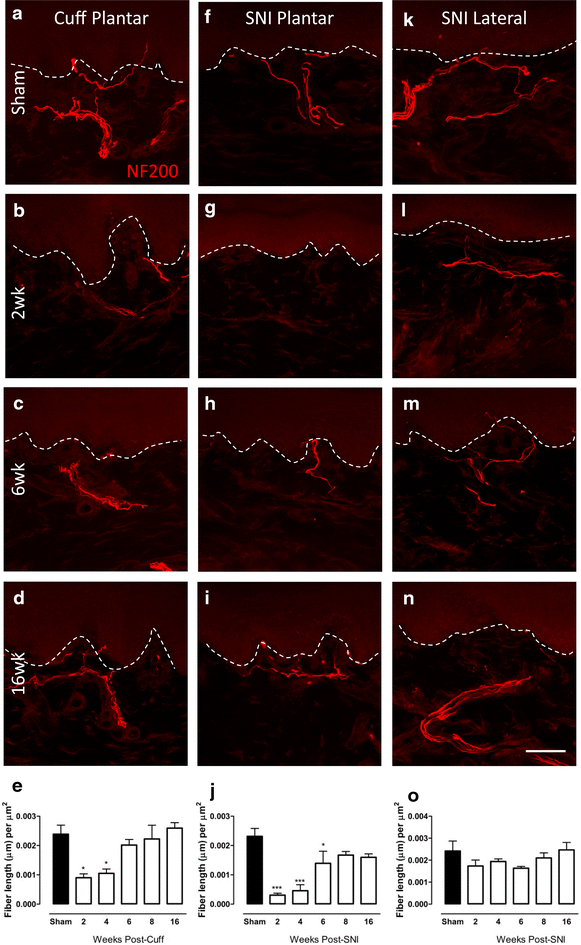
Changes in NF200-IR fibre innervation in the upper dermis of the paw skin of cuff and SNI rats. Photomicrographs show representative examples of NF200-IR fibre innervation (red) in the plantar paw skin of sham animals (a), and 2 (b), 6 (c) and 16 weeks (d) after cuff. Representative images of NF200-IR innervation in the plantar paw skin of sham animals (f) and 2 (g), 6 (h) and 16 (i) weeks after SNI. Representative images of NF200-IR innervation in the lateral paw skin of sham animals (k) and 2 (l), 6 (m) and 16 (n) weeks after SNI. Bar graphs show average NF200-IR fibre length (µm) per unit area of upper dermis (µm2) in the plantar paw skin at various times after cuff (e) and SNI (j) and in the lateral paw skin after SNI (o). Each point represents the mean ± SEM (n = 4–6 per group); *p < 0.05, ***p < 0.001 by one way ANOVA with Dunnett’s post hoc; scale bar 50 µm
Fig. 3
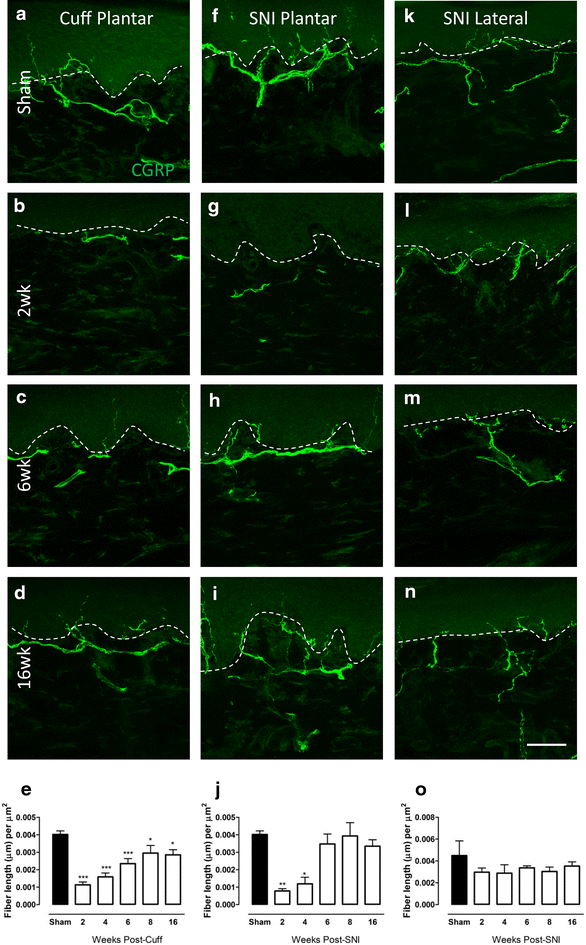
Changes in CGRP-IR fibre innervation in the upper dermis of the paw skin of cuff and SNI rats. Photomicrographs show representative examples of CGRP-IR fibre innervation (green) in the plantar paw skin of sham animals (a), and 2 (b), 6 (c) and 16 weeks (d) after cuff. Representative images of CGPR-IR innervation in the plantar paw skin of sham animals (f) and 2 (g), 6 (h) and 16 (i) weeks after SNI. Representative images of CGRP-IR innervation in the lateral paw skin of sham animals (k) and 2 (l), 6 (m) and 16 (n) weeks after SNI. Bar graphs show average CGRP-IR fibre length (µm) per unit area of upper dermis (µm2) in the plantar paw skin at various times after cuff (e), SNI (j) and in the lateral paw skin after SNI (o). Each point represents the mean ± SEM (n = 4–6 per group); *p < 0.05, ***p < 0.001 by one way ANOVA with Dunnett’s post hoc; scale bar 50 µm
Fig. 4
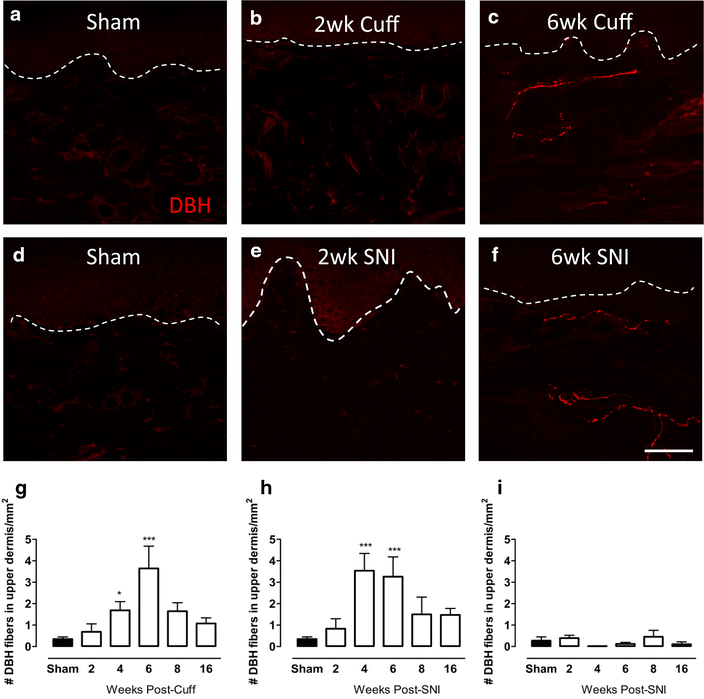
Changes in DBH-IR sympathetic fibre innervation in the upper dermis of the paw skin of cuff and SNI rats. Photomicrographs show representative examples of DBH-IR fibre innervation (red) in the plantar paw skin of sham animals and 2, 6 weeks after cuff (a–c) or SNI (d–f). Bar graphs show average number of ectopic DBH-IR fibres in the upper dermis of the plantar paw skin of cuff (g) and SNI rats (h) and in the lateral paw skin of SNI animals (i) at various times after injury. The values reported are per 1 mm2 of upper dermis. Each point represents the mean ± SEM (n = 4–6 per group); *p < 0.05, ***p < 0.001 by one way ANOVA with Dunnett’s post hoc; scale bar 50 µm
Fig. 5
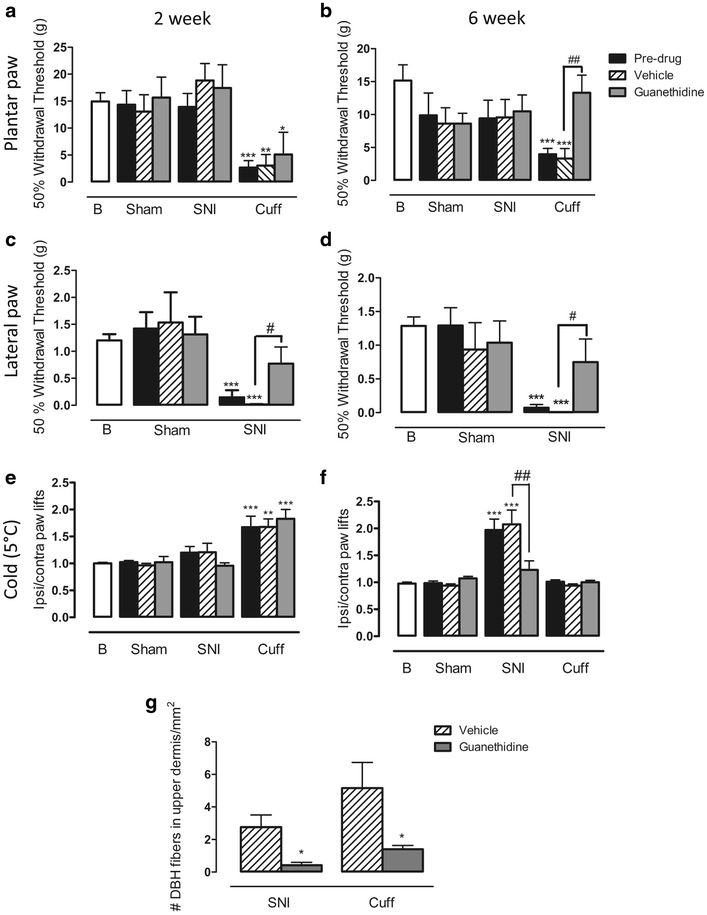
The effect of chemical sympathectomy with guanethidine on behavioural signs of pain. a 50 % withdrawal threshold to von Frey fibres in the plantar paw of cuff, SNI, and sham rats treated with guanethidine or vehicle 2 weeks after surgery. Cuff animals had significant mechanical allodynia, and this was unaltered by guanethidine. b 50 % withdrawal threshold to von Frey fibres in the plantar paw of cuff, SNI, and sham rats treated with guanethidine or vehicle 6 weeks after surgery. Cuff animals had significant mechanical allodynia which was completely alleviated by guanethidine. c 50 % withdrawal thresholds to von Frey fibres in the lateral paw of SNI, and sham rats treated with guanethidine or vehicle 2 weeks after surgery. SNI animals had significant mechanical allodynia which was partially alleviated by guanethidine. d 50 % withdrawal thresholds to von Frey fibres in the lateral paw of SNI, and sham rats treated with guanethidine or vehicle 6 weeks after surgery. SNI animals had significant mechanical allodynia which was partially alleviated by guanethidine. e Responses to cold plate set to 5 °C in cuff, SNI and sham rats treated with guanethidine or vehicle 2 weeks after surgery. Cuff animals had significant cold hyperalgesia which was unaltered by guanethidine. f Responses to cold plate set to 5 °C in cuff, SNI and sham rats treated with guanethidine or vehicle 6 weeks after surgery. SNI animals had significant cold hyperalgesia which was reduced by guanethidine. Each point represents the mean ± SEM (n = 6–8 per group). B baseline. *p < 0.05, **p < 0.01, ***p < 0.001 compared with baseline, #p < 0.05, ##p < 0.01 compared to vehicle treated rats, by a one way ANOVA with Bonferroni post hoc. g Bar graph showing the mean number of DBH-IR fibres in the upper dermis per 1 mm2 in 6 week cuff and SNI animals after guanethidine or vehicle. *p < 0.05 by t-test
Fig. 6
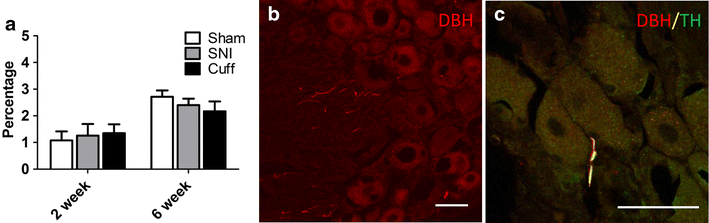
Sympathetic fibres do not sprout in the DRG of cuff and SNI animals. a Bar graph showing the percentage of cells in close proximity to a DBH-IR sympathetic fibre in the DRG at 2 and 6 weeks after cuff and SNI. b Photomicrograph showing representative DBH-IR fibres in the DRG of a 6 week cuff rat. Note that while sympathetic fibres can be seen within the DRG, they are not in close contact with cell bodies and the pattern of staining is no different in sham animals. c TH and DBH immunoreactivities were fully colocalized in sympathetic fibres in the DRG; this micrograph shows a rare TH-IR (green) + DBH-IR (red) sympathetic fibre within the DRG in close proximity to a cell body in a 6 week cuff rat. For quantitative purposes n = 3–4 per group. Scale bar 50 µm
Similar articles
- Delayed sympathetic dependence in the spared nerve injury (SNI) model of neuropathic pain.
Pertin M, Allchorne AJ, Beggah AT, Woolf CJ, Decosterd I. Pertin M, et al. Mol Pain. 2007 Jul 31;3:21. doi: 10.1186/1744-8069-3-21. Mol Pain. 2007. PMID: 17672895 Free PMC article. - Sympathetic sprouting and changes in nociceptive sensory innervation in the glabrous skin of the rat hind paw following partial peripheral nerve injury.
Yen LD, Bennett GJ, Ribeiro-da-Silva A. Yen LD, et al. J Comp Neurol. 2006 Apr 20;495(6):679-90. doi: 10.1002/cne.20899. J Comp Neurol. 2006. PMID: 16506190 - Delayed reinnervation by nonpeptidergic nociceptive afferents of the glabrous skin of the rat hindpaw in a neuropathic pain model.
Peleshok JC, Ribeiro-da-Silva A. Peleshok JC, et al. J Comp Neurol. 2011 Jan 1;519(1):49-63. doi: 10.1002/cne.22500. J Comp Neurol. 2011. PMID: 21120927 - Postsympathectomy pain and changes in sensory neuropeptides: towards an animal model.
Schon F. Schon F. Lancet. 1985 Nov 23;2(8465):1158-60. doi: 10.1016/s0140-6736(85)92682-0. Lancet. 1985. PMID: 2414615 Review. - Post-sympathectomy neuralgia: hypotheses on peripheral and central neuronal mechanisms.
Kramis RC, Roberts WJ, Gillette RG. Kramis RC, et al. Pain. 1996 Jan;64(1):1-9. doi: 10.1016/0304-3959(95)00060-7. Pain. 1996. PMID: 8867242 Review.
Cited by
- The neuropathic phenotype of the K/BxN transgenic mouse with spontaneous arthritis: pain, nerve sprouting and joint remodeling.
Gonçalves Dos Santos G, Jimenéz-Andrade JM, Woller SA, Muñoz-Islas E, Ramírez-Rosas MB, Ohashi N, Ferreira Catroli G, Fujita Y, Yaksh TL, Corr M. Gonçalves Dos Santos G, et al. Sci Rep. 2020 Sep 24;10(1):15596. doi: 10.1038/s41598-020-72441-5. Sci Rep. 2020. PMID: 32973194 Free PMC article. - Localized sympathectomy reduces peripheral nerve regeneration and pain behaviors in 2 rat neuropathic pain models.
Xie W, Strong JA, Zhang JM. Xie W, et al. Pain. 2020 Aug;161(8):1925-1936. doi: 10.1097/j.pain.0000000000001887. Epub 2020 Apr 16. Pain. 2020. PMID: 32701850 Free PMC article. - Referred Somatic Hyperalgesia Mediates Cardiac Regulation by the Activation of Sympathetic Nerves in a Rat Model of Myocardial Ischemia.
Cui X, Sun G, Cao H, Liu Q, Liu K, Wang S, Zhu B, Gao X. Cui X, et al. Neurosci Bull. 2022 Apr;38(4):386-402. doi: 10.1007/s12264-022-00841-w. Epub 2022 Apr 26. Neurosci Bull. 2022. PMID: 35471719 Free PMC article. - Peripheral Mechanisms of Neuropathic Pain-the Role of Neuronal and Non-Neuronal Interactions and Their Implications for Topical Treatment of Neuropathic Pain.
Kocot-Kępska M, Zajączkowska R, Mika J, Wordliczek J, Dobrogowski J, Przeklasa-Muszyńska A. Kocot-Kępska M, et al. Pharmaceuticals (Basel). 2021 Jan 20;14(2):77. doi: 10.3390/ph14020077. Pharmaceuticals (Basel). 2021. PMID: 33498496 Free PMC article. Review. - NR2B Expression in Rat DRG Is Differentially Regulated Following Peripheral Nerve Injuries That Lead to Transient or Sustained Stimuli-Evoked Hypersensitivity.
Norcini M, Sideris A, Adler SM, Hernandez LA, Zhang J, Blanck TJ, Recio-Pinto E. Norcini M, et al. Front Mol Neurosci. 2016 Oct 18;9:100. doi: 10.3389/fnmol.2016.00100. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27803647 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous