Trafficking regulates the subcellular distribution of voltage-gated sodium channels in primary sensory neurons - PubMed (original) (raw)
Review
Trafficking regulates the subcellular distribution of voltage-gated sodium channels in primary sensory neurons
Lan Bao. Mol Pain. 2015.
Abstract
Voltage-gated sodium channels (Navs) comprise at least nine pore-forming α subunits. Of these, Nav1.6, Nav1.7, Nav1.8 and Nav1.9 are the most frequently studied in primary sensory neurons located in the dorsal root ganglion and are mainly localized to the cytoplasm. A large pool of intracellular Navs raises the possibility that changes in Nav trafficking could alter channel function. The molecular mediators of Nav trafficking mainly consist of signals within the Navs themselves, interacting proteins and extracellular factors. The surface expression of Navs is achieved by escape from the endoplasmic reticulum and proteasome degradation, forward trafficking and plasma membrane anchoring, and it is also regulated by channel phosphorylation and ubiquitination in primary sensory neurons. Axonal transport and localization of Navs in afferent fibers involves the motor protein KIF5B and scaffold proteins, including contactin and PDZ domain containing 2. Localization of Nav1.6 to the nodes of Ranvier in myelinated fibers of primary sensory neurons requires node formation and the submembrane cytoskeletal protein complex. These findings inform our understanding of the molecular and cellular mechanisms underlying Nav trafficking in primary sensory neurons.
Figures
Fig. 1
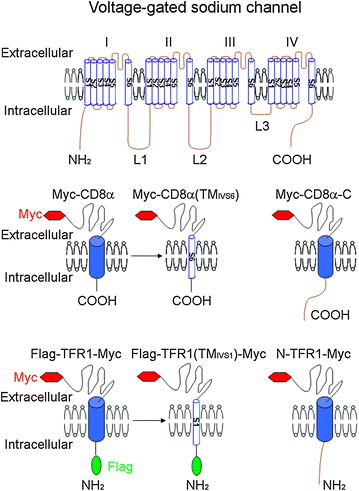
Model molecules to identify the signals in Navs that mediate the trafficking regulation. Navs consist of four domains (I, II, III and IV) connected by three intracellular loops (L1–L3); each domain is formed by six transmembrane segments (TM; S1–S6). Both the N-terminus (N) and the C-terminus (C) of Navs are located in the cytoplasm. CD8α and TFR1, which have distinct cell-surface localization, are adapted to detect the roles that particular regions of the Navs have in subcellular distribution. The type I membrane protein CD8α is suitable for testing three intracellular loops, the C-terminus and the transmembrane segments that pass through the membrane in the extracellular to intracellular direction (S2, S4 and S6), whereas the type II membrane protein TFR1 is appropriate for testing the N-terminus and the transmembrane segments that pass through the membrane in the opposite direction (S1, S3 and S5). A Myc tag is inserted to the N-terminus of CD8α or the C-terminus of TFR1, and non-permeabilized immunostaining is performed with Myc antibody in transfected living cells to label these proteins on the plasma membrane. A Flag tag is inserted to the N-terminus of TFR1 for the permeabilized immunostaining with Flag antibody to label the protein in whole cell. The sequence of CD8α or TFR1 is replaced with corresponding region of Nav, such as Myc-CD8α(TMIVS6), Myc-CD8α-C, Flag-TFR1(TMIVS1)-Myc and N-TFR1-Myc. This figure is adapted from Li et al. [15]
Fig. 2
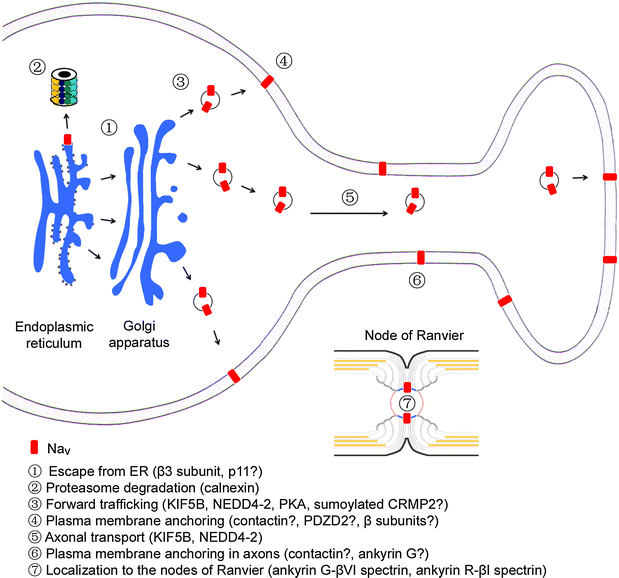
Main steps that regulates the subcellular distribution of Navs in primary sensory neurons. The surface expression of Navs is achieved by escape from the endoplasmic reticulum and proteasome degradation, forward trafficking and plasma membrane anchoring in primary sensory neurons. Axonal transport and localization of Navs in afferent fibers involves motor proteins and scaffold proteins. Localization of Nav1.6 to the nodes of Ranvier in myelinated fibers of primary sensory neurons requires node formation and the submembrane cytoskeletal protein complex. The molecules listed are mostly positive regulators except NEDD4-2 that may impede forward trafficking of Nav1.7. However, the hypothesized roles of molecules with question mark during various steps of Nav trafficking in primary sensory neuron need to be proved
Similar articles
- Distribution of TTX-sensitive voltage-gated sodium channels in primary sensory endings of mammalian muscle spindles.
Carrasco DI, Vincent JA, Cope TC. Carrasco DI, et al. J Neurophysiol. 2017 Apr 1;117(4):1690-1701. doi: 10.1152/jn.00889.2016. Epub 2017 Jan 25. J Neurophysiol. 2017. PMID: 28123009 Free PMC article. - Regulation of cough and action potentials by voltage-gated Na channels.
Carr MJ. Carr MJ. Pulm Pharmacol Ther. 2013 Oct;26(5):508-9. doi: 10.1016/j.pupt.2013.07.001. Epub 2013 Jul 11. Pulm Pharmacol Ther. 2013. PMID: 23850655 - CRMP2 protein SUMOylation modulates NaV1.7 channel trafficking.
Dustrude ET, Wilson SM, Ju W, Xiao Y, Khanna R. Dustrude ET, et al. J Biol Chem. 2013 Aug 23;288(34):24316-31. doi: 10.1074/jbc.M113.474924. Epub 2013 Jul 8. J Biol Chem. 2013. PMID: 23836888 Free PMC article. - Trafficking mechanisms underlying Nav channel subcellular localization in neurons.
Solé L, Tamkun MM. Solé L, et al. Channels (Austin). 2020 Dec;14(1):1-17. doi: 10.1080/19336950.2019.1700082. Channels (Austin). 2020. PMID: 31841065 Free PMC article. Review. - Blocking voltage-gated sodium channels as a strategy to suppress pathological cough.
Sun H, Kollarik M, Undem BJ. Sun H, et al. Pulm Pharmacol Ther. 2017 Dec;47:38-41. doi: 10.1016/j.pupt.2017.05.010. Epub 2017 May 15. Pulm Pharmacol Ther. 2017. PMID: 28522215 Review.
Cited by
- Magi-1 scaffolds NaV1.8 and Slack KNa channels in dorsal root ganglion neurons regulating excitability and pain.
Pryce KD, Powell R, Agwa D, Evely KM, Sheehan GD, Nip A, Tomasello DL, Gururaj S, Bhattacharjee A. Pryce KD, et al. FASEB J. 2019 Jun;33(6):7315-7330. doi: 10.1096/fj.201802454RR. Epub 2019 Mar 12. FASEB J. 2019. PMID: 30860870 Free PMC article. - Mapping protein interactions of sodium channel NaV1.7 using epitope-tagged gene-targeted mice.
Kanellopoulos AH, Koenig J, Huang H, Pyrski M, Millet Q, Lolignier S, Morohashi T, Gossage SJ, Jay M, Linley JE, Baskozos G, Kessler BM, Cox JJ, Dolphin AC, Zufall F, Wood JN, Zhao J. Kanellopoulos AH, et al. EMBO J. 2018 Feb 1;37(3):427-445. doi: 10.15252/embj.201796692. Epub 2018 Jan 15. EMBO J. 2018. PMID: 29335280 Free PMC article. - Interaction of drugs with lipid raft membrane domains as a possible target.
Tsuchiya H, Mizogami M. Tsuchiya H, et al. Drug Target Insights. 2020 Dec 22;14:34-47. doi: 10.33393/dti.2020.2185. eCollection 2020. Drug Target Insights. 2020. PMID: 33510571 Free PMC article. Review. - Anterograde Axonal Transport in Neuronal Homeostasis and Disease.
Guillaud L, El-Agamy SE, Otsuki M, Terenzio M. Guillaud L, et al. Front Mol Neurosci. 2020 Sep 18;13:556175. doi: 10.3389/fnmol.2020.556175. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33071754 Free PMC article. Review. - Expression and Role of Voltage-Gated Sodium Channels in Human Dorsal Root Ganglion Neurons with Special Focus on Nav1.7, Species Differences, and Regulation by Paclitaxel.
Chang W, Berta T, Kim YH, Lee S, Lee SY, Ji RR. Chang W, et al. Neurosci Bull. 2018 Feb;34(1):4-12. doi: 10.1007/s12264-017-0132-3. Epub 2017 Apr 19. Neurosci Bull. 2018. PMID: 28424991 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous