Complement Evasion Mediated by Enhancement of Captured Factor H: Implications for Protection of Self-Surfaces from Complement - PubMed (original) (raw)
Complement Evasion Mediated by Enhancement of Captured Factor H: Implications for Protection of Self-Surfaces from Complement
Andrew P Herbert et al. J Immunol. 2015.
Abstract
In an attempt to evade annihilation by the vertebrate complement system, many microbes capture factor H (FH), the key soluble complement-regulating protein in human plasma. However, FH is normally an active complement suppressor exclusively on self-surfaces and this selective action of FH is pivotal to self versus non-self discrimination by the complement system. We investigated whether the bacterially captured FH becomes functionally enhanced and, if so, how this is achieved at a structural level. We found, using site-directed and truncation mutagenesis, surface plasmon resonance, nuclear magnetic resonance spectroscopy, and cross-linking and mass spectrometry, that the N-terminal domain of Streptococcus pneumoniae protein PspC (PspCN) not only binds FH extraordinarily tightly but also holds it in a previously uncharacterized conformation. Functional enhancement arises from exposure of a C-terminal cryptic second binding site in FH for C3b, the activation-specific fragment of the pivotal complement component, C3. This conformational change of FH doubles its affinity for C3b and increases 5-fold its ability to accelerate decay of the binary enzyme (C3bBb) responsible for converting C3 to C3b in an amplification loop. Despite not sharing critical FH-binding residues, PspCNs from D39 and Tigr4 S. pneumoniae exhibit similar FH-anchoring and enhancing properties. We propose that these bacterial proteins mimic molecular markers of self-surfaces, providing a compelling hypothesis for how FH prevents complement-mediated injury to host tissue while lacking efficacy on virtually all other surfaces. In hemolysis assays with 2-aminoethylisothiouronium bromide-treated erythrocytes that recapitulate paroxysmal nocturnal hemoglobinuria, PspCN enhanced protection of cells by FH, suggesting a new paradigm for therapeutic complement suppression.
Copyright © 2015 The Authors.
Figures
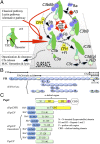
FIGURE 1.
Proteins used in this study. (A) The alternative pathway produces, spontaneously, low levels of C3b from inert C3. Nascent C3b can attach to nearby surfaces via its TED. C3b also binds FB, forming C3bB. Factor D (FD) cleaves the FB portion of C3bB, yielding C3bBb. C3bBb converts C3 to C3b (+ C3a), establishing a positive-feedback loop for C3b production. On a self-surface, FH competes with FB for C3b binding and accelerates decay of C3bBb. C3b can be degraded to iC3b by FI with FH as cofactor and then via C3dg (+ C3c) to C3d, equating to the TED of C3b. (B) Ovals represent CCPs 1–20 in FH; locations of N-glycans are indicated by rectangles. Horizontal lines above the ovals designate CCPs involved in binding C3b (ovals with thicker outline), self-surface markers (pale shading), and C3d (pale shading plus thicker outline); dashed lines indicate constitutively occluded binding sites. (C) N-terminally His6-tagged SUMO fusions (s) with sequences from the N-terminal domain of D39 PspC (residue numbering includes signaling sequence) were created. The sPspCN37–140 or the product (PspCN) following cleavage of and removal of the fusion partner was used for most experiments.
FIGURE 2.
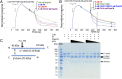
FH binds irreversibly to (D39)PspCN. (A) A 2-fold dilution series, from 8 to 0.25 nM, of plasma-purified human FH was flowed (in duplicate) over D39 His6-Sumo-PspCN immobilized (to Ni-NTA) in an SPR experiment (see also Supplemental Fig. 1). (B) The experiments shown in (A) were repeated with recombinant wild-type FH (left) or rFH(D1119G) (right). In (A) and (B), solid black lines indicate fitted curves. (C) A 2-fold dilution series from 40 to 2.5 nM of FH was flowed (in duplicate) over recombinant sPspCN truncations (as indicated using residue numbers, see Fig. 1C) immobilized on Ni-NTA. Residues 61–140 represent the minimum region of D39 PspC shown in the present study to bind tightly to FH, but (D39)PspC37–140 (i.e., PspCN) was used for further experiments owing to its greater stability.
FIGURE 3.
(D39)PspCN binds FH 8–9 but additional CCPs are essential for irreversible binding. D39 sPspCN was immobilized as in Fig. 2A. (A) Comparison of binding curves obtained for 8 nM FH as well as 50 nM FH 8–9, FH 8–15, FH 10–15 (near baseline), or FH 19–20 (just above baseline). (B) Expanded segment of the sensorgram in (A) highlighting small responses for FH 19–20 and FH 10–15 (_K_D values could not be estimated). (C) An FH 8–9 concentration series was flowed over immobilized D39 sPspCN to allow estimates (equilibrium method) of _K_D of 30 nM. Another series for FH 8–15 (not shown) yielded a _K_D of 1 nM.
FIGURE 4.
NMR-based study of FH 8-9:PspCN complex. 1H,15H(NMR) spectra for (A) free D39 15N-PspCN, (B) D39 15N-PspCN bound to FH 8–9, (C) free 15N-FH 8–9, (D) 15N-FH 8–9 bound to (D39)PspCN. Differences between (A) and (B) suggest D39(PspCN) adopts a more compactly folded structure upon binding FH 8–9. Many chemical shift differences between (C) and (D) suggest extensive contacts between FH 8–9 and (D39)PspCN. For higher resolution and overlaid spectra (see Supplemental Fig. 2).
FIGURE 5.
Architecture and oligomeric status of FH:PspCN complexes. (A) Cross-linked products were resolved by SDS-PAGE. Lane 1, molecular mass markers; lanes 2 and 3, cross-linker (BS3) mixed with FH (0.5:1 BS3/protein molar ratio); lanes 5 and 6, as lanes 2 and 3 but 4:1 BS3/protein molar ratio; lanes 8 and 9, BS3 mixed with FH:(D39)PspCN complex (4:1 BS3/protein complex molar ratio). Coomassie-stained bands (labeled s1–s5) were cut out for MS/MS analysis (Supplemental Table I): s1 and s2 correspond to distinct monomeric species with different migration rates, s3 corresponds to a cross-linked dimer, and s4 and s5 correspond to a cross-linked FH monomer and dimer, respectively, in complex with (D39)PspCN. (B) In cartoons of FH, numbered ovals indicate CCPs and dotted connectors represent cross-linked peptide pairs detected by MS/MS in analysis of SDS-PAGE bands corresponding to monomeric FH (s1 and s2) or the 1:1 FH:PspCN complex s4. Connectors are black when they represent cross-links that are observed equally in all three bands (s1, s2, and s4) or colored when they are selectively enriched in one or two bands (see key, Materials and Methods and Supplemental Table I). (C) Size-exclusion chromatograms and multiple-angle light scattering–derived molecular masses for FH and FH:(D39)PspCN complex. The 155-kDa value for FH matches the calculated monomeric molecular mass. Material in the main FH:PspCN peak had a mean estimated molecular mass of 172 kDa, close to that expected for 1:1 (167 kDa). The FH:PspCN peak has a trailing shoulder attributable to nonspecific association with resin. A minor peak (molecular mass of 189 kDa) could represent some 1:2 or 1:3 FH:PspCN complexes, but there was no evidence of complexes containing two molecules of FH (≥310 kDa). (D) Analytical ultracentrifugation of free FH showed a single species; FH:(D39)PspCN also behaved in analytical ultracentrifugation as a single species with a higher Svedberg value than free FH.
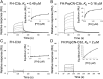
FIGURE 6.
Binding properties of wild-type (plasma-derived) FH with and without D39 PspCN. (A and B) Demonstration by SPR that FH (A) has lower affinity than does the FH:(D39)PspCN complex (B) for immobilized C3b (amine coupled on a C1 chip). (C and D) The lower affinity of FH for C3b may arise from FH (C) having a very substantially lower affinity than the FH:(D39)PspCN complex (D) for immobilized C3d amine coupled to a different channel of the same C1 chip as used in (A) and (B).
FIGURE 7.
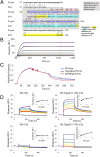
Functional properties of wild-type and mutant FH with and without D39 PspCN. (A) Formation of C3bBb from C3b, FB, and FD on the chip surface (see Fig. 1A) and subsequent loss of Bb, monitored by SPR. Curves are color coded (see key). Note that 100 nM PspCN alone has no activity; 5 nM FH:(D39)PspCN complex accelerated decay better than did 10 nM FH alone. (B) Incrementally increasing FH from 10 to 80 nM correlates with greater fluid-phase FI cofactor activity, judging by stronger bands representing the C3b α′-chain cleavage products at 39 and 63 kDa, and loss of intensity of the band corresponding to intact α′-chain. PspCN lacks intrinsic cofactor activity whereas binding of PspCN to FH does not enhance its activity. (C) In a parallel experiment to that of (A), 5 nM FH(D1119G) and 5 nM wild-type FH have similar DAAs but (D39)PspCN enhances the activity of wild-type FH more than it enhances the activity of FH(D1119G).
FIGURE 8.
(Tigr4)PspC37–179 shares FH binding and activating properties with (D39)PspCN. (A) Pairwise (BLAST-P) alignment of N-terminal sequences from D39 and Tigr4 PspC. The sequence of (D39)PspCN (i.e., residues 37–140) is compared with that of (Tigr)PspC37–179. The segment of Tigr4 PspCN (residues 68–140) used in a recent crystal structure (48) of a complex with FH CCP 9 (PDB_ID = 4k12) is shaded in gray (boxes indicate α-helices); yellow and blue highlighting of residues summarizes information from truncation mutants in Fig. 2C. Intermolecular interface residues are indicated (red signifies >60% buried) as “X” or “h” (when H-bonded) or “H” if H-bonded and in salt bridge); *, key interface residue that is not conserved (analyzed using PISA) (54). (B) In this SPR-based experiment, a 2-fold dilution series of FH solution from 16 to 0.25 nM was flowed over immobilized (Tigr4)PspC37–179, suggesting that binding is irreversible. (C) A complex of (Tigr4)PspC37–179 and FH is more effective than FH alone at accelerating decay of C3bBb, but not as effective in this respect as (D39)PspCN:FH. In these experiments, 20 nM FH was used. (D) (Tigr4)PspCN enhances FH binding to C3b and C3d. In measurements that were analogous to those of Fig. 6, solutions of either FH (left), or FH:(Tigr4)PspCN37–179 (right) (at FH 2-fold dilutions from 1000 to 31 nM in both cases) were flowed over C3b or C3d amine coupled to the SPR chip. Derived _K_D values are reported in Table I.
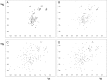
FIGURE 9.
Binding properties of FH(D1119G) with and without (D39)PspCN. (A) According to SPR, FH(D1119G) binds to immobilized C3b with a similar _K_D value to that of wild-type FH. (B) Importantly, there is little enhancement of C3b binding when FH(D1119G) is complexed with (D39)PspCN. Neither (C) FH(D1119G) alone nor (D) FH(D1119G):PspCN bind well to C3d. Insets, Plots to estimate _K_Ds.
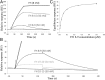
FIGURE 10.
Cell-based assays. (A) Near-complete human complement-mediated lysis (after 30 min) of rabbit erythrocytes, measured by A412 of the released hemoglobin at 40% (v/v) FHΔNHS. Neither FH, nor FH(D1119G), nor 1:1 FH+FH(D1119G) protected rabbit erythrocytes significantly. (D39)PspCN:FH(wild-type), but not (D39)PspCN:FH(D1119G), affords some, although incomplete, protection. (B) As expected, FH prevents human complement-mediated hemolysis of (human-like) sheep erythrocytes irrespective of (D39)PspCN, whereas FH(D1119G) provides less protection than does wild-type FH. Protection of sheep erythrocytes by the 1:1 mixture of FH(wild-type) plus FH(D1119G), recapitulating heterozygosity, is enhanced by PspCN. (C and D) (D39)PspCN boosts ability of FH in serum to prevent hemolysis of PNH-like human erythrocytes (treated with AET to deprive them of decay-accelerating factor). These AET-treated cells are susceptible to acidified serum lysis in a similar way to erythrocytes from PNH patients. (C) Adding increasing concentrations of FH inhibits lysis of the PNH-like erythrocytes. (D) Adding increasing concentrations of (D39)PspCN inhibits lysis of the PNH-like erythrocytes with an IC50 of 28 nM.
FIGURE 11.
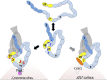
Model for functional enhancement of FH by self-surface markers and PspCN. Ovals represent CCPs; selected cross-links are shown (see Fig. 5B). Circulating FH (center panels) adopts extended conformations (from band s1 in Fig. 5A), not conducive to cross-links between non-neighboring CCPs. Also adopted are more compact conformations that are captured by cross-linking (from band s2 in Fig. 5A). We cannot tell which of these prevail under physiological conditions. PspCN stabilizes FH conformers that resemble the bent-back ones in s2 but that produce different cross-linking patterns. The PspCN-stabilized FH conformers (bottom left) bind better to C3b and C3d. Rearrangements of CCPs 7 and 20 probably contribute to functional enhancement, as this is where most differences in cross-linking occur. These two CCPs also bind to GAGs and sialic acid and to numerous other bacterial proteins. This suggests that engagement with these markers of self-surfaces may induce a functionally enhanced conformation of FH (bottom right) similar to the one captured by cross-linking in band s4 of Fig. 5A.
Similar articles
- Streptococcus pneumoniae capsular serotype invasiveness correlates with the degree of factor H binding and opsonization with C3b/iC3b.
Hyams C, Trzcinski K, Camberlein E, Weinberger DM, Chimalapati S, Noursadeghi M, Lipsitch M, Brown JS. Hyams C, et al. Infect Immun. 2013 Jan;81(1):354-63. doi: 10.1128/IAI.00862-12. Epub 2012 Nov 12. Infect Immun. 2013. PMID: 23147038 Free PMC article. - Staphylococcal Ecb protein and host complement regulator factor H enhance functions of each other in bacterial immune evasion.
Amdahl H, Jongerius I, Meri T, Pasanen T, Hyvärinen S, Haapasalo K, van Strijp JA, Rooijakkers SH, Jokiranta TS. Amdahl H, et al. J Immunol. 2013 Aug 15;191(4):1775-84. doi: 10.4049/jimmunol.1300638. Epub 2013 Jul 17. J Immunol. 2013. PMID: 23863906 - Disease-linked mutations in factor H reveal pivotal role of cofactor activity in self-surface-selective regulation of complement activation.
Kerr H, Wong E, Makou E, Yang Y, Marchbank K, Kavanagh D, Richards A, Herbert AP, Barlow PN. Kerr H, et al. J Biol Chem. 2017 Aug 11;292(32):13345-13360. doi: 10.1074/jbc.M117.795088. Epub 2017 Jun 21. J Biol Chem. 2017. PMID: 28637873 Free PMC article. - Functional anatomy of complement factor H.
Makou E, Herbert AP, Barlow PN. Makou E, et al. Biochemistry. 2013 Jun 11;52(23):3949-62. doi: 10.1021/bi4003452. Epub 2013 May 31. Biochemistry. 2013. PMID: 23701234 Review.
Cited by
- Murine Factor H Co-Produced in Yeast With Protein Disulfide Isomerase Ameliorated C3 Dysregulation in Factor H-Deficient Mice.
Kerr H, Herbert AP, Makou E, Abramczyk D, Malik TH, Lomax-Browne H, Yang Y, Pappworth IY, Denton H, Richards A, Marchbank KJ, Pickering MC, Barlow PN. Kerr H, et al. Front Immunol. 2021 May 12;12:681098. doi: 10.3389/fimmu.2021.681098. eCollection 2021. Front Immunol. 2021. PMID: 34054871 Free PMC article. - Complement Evasion Strategies of Human Pathogenic Bacteria.
Sharma S, Bhatnagar R, Gaur D. Sharma S, et al. Indian J Microbiol. 2020 Sep;60(3):283-296. doi: 10.1007/s12088-020-00872-9. Epub 2020 Apr 24. Indian J Microbiol. 2020. PMID: 32655196 Free PMC article. Review. - The Role of the Complement System in the Pathogenesis of Infectious Forms of Hemolytic Uremic Syndrome.
Avdonin PP, Blinova MS, Generalova GA, Emirova KM, Avdonin PV. Avdonin PP, et al. Biomolecules. 2023 Dec 27;14(1):39. doi: 10.3390/biom14010039. Biomolecules. 2023. PMID: 38254639 Free PMC article. Review. - Tuning the Functionality by Splicing: Factor H and Its Alternative Splice Variant FHL-1 Share a Gene but Not All Functions.
Mannes M, Dopler A, Huber-Lang M, Schmidt CQ. Mannes M, et al. Front Immunol. 2020 Oct 15;11:596415. doi: 10.3389/fimmu.2020.596415. eCollection 2020. Front Immunol. 2020. PMID: 33178228 Free PMC article. Review. - Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition.
Yu J, Yuan X, Chen H, Chaturvedi S, Braunstein EM, Brodsky RA. Yu J, et al. Blood. 2020 Oct 29;136(18):2080-2089. doi: 10.1182/blood.2020008248. Blood. 2020. PMID: 32877502 Free PMC article.
References
- Lachmann P. J. 2009. The amplification loop of the complement pathways. Adv. Immunol. 104: 115–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G1001971/MRC_/Medical Research Council/United Kingdom
- 091020/WT_/Wellcome Trust/United Kingdom
- 103139/Z/13/Z/WT_/Wellcome Trust/United Kingdom
- 092076/WT_/Wellcome Trust/United Kingdom
- BB/I007946/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 081179/WT_/Wellcome Trust/United Kingdom
- 103139/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous