Molecular Heterogeneity and Response to Neoadjuvant Human Epidermal Growth Factor Receptor 2 Targeting in CALGB 40601, a Randomized Phase III Trial of Paclitaxel Plus Trastuzumab With or Without Lapatinib - PubMed (original) (raw)
Clinical Trial
. 2016 Feb 20;34(6):542-9.
doi: 10.1200/JCO.2015.62.1268. Epub 2015 Nov 2.
Donald A Berry 2, Constance T Cirrincione 2, William T Barry 2, Brandelyn N Pitcher 2, Lyndsay N Harris 2, David W Ollila 2, Ian E Krop 2, Norah Lynn Henry 2, Douglas J Weckstein 2, Carey K Anders 2, Baljit Singh 2, Katherine A Hoadley 2, Michael Iglesia 2, Maggie Chon U Cheang 2, Charles M Perou 2, Eric P Winer 2, Clifford A Hudis 2
Affiliations
- PMID: 26527775
- PMCID: PMC4980567
- DOI: 10.1200/JCO.2015.62.1268
Clinical Trial
Molecular Heterogeneity and Response to Neoadjuvant Human Epidermal Growth Factor Receptor 2 Targeting in CALGB 40601, a Randomized Phase III Trial of Paclitaxel Plus Trastuzumab With or Without Lapatinib
Lisa A Carey et al. J Clin Oncol. 2016.
Abstract
Purpose: Dual human epidermal growth factor receptor 2 (HER2) targeting can increase pathologic complete response rates (pCRs) to neoadjuvant therapy and improve progression-free survival in metastatic disease. CALGB 40601 examined the impact of dual HER2 blockade consisting of trastuzumab and lapatinib added to paclitaxel, considering tumor and microenvironment molecular features.
Patients and methods: Patients with stage II to III HER2-positive breast cancer underwent tumor biopsy followed by random assignment to paclitaxel plus trastuzumab alone (TH) or with the addition of lapatinib (THL) for 16 weeks before surgery. An investigational arm of paclitaxel plus lapatinib (TL) was closed early. The primary end point was pCR in the breast; correlative end points focused on molecular features identified by gene expression-based assays.
Results: Among 305 randomly assigned patients (THL, n = 118; TH, n = 120; TL, n = 67), the pCR rate was 56% (95% CI, 47% to 65%) with THL and 46% (95% CI, 37% to 55%) with TH (P = .13), with no effect of dual therapy in the hormone receptor-positive subset but a significant increase in pCR with dual therapy in those with hormone receptor-negative disease (P = .01). The tumors were molecularly heterogeneous by gene expression analysis using mRNA sequencing (mRNAseq). pCR rates significantly differed by intrinsic subtype (HER2 enriched, 70%; luminal A, 34%; luminal B, 36%; P < .001). In multivariable analysis treatment arm, intrinsic subtype, HER2 amplicon gene expression, p53 mutation signature, and immune cell signatures were independently associated with pCR. Post-treatment residual disease was largely luminal A (69%).
Conclusion: pCR to dual HER2-targeted therapy was not significantly higher than single HER2 targeting. Tissue analysis demonstrated a high degree of intertumoral heterogeneity with respect to both tumor genomics and tumor microenvironment that significantly affected pCR rates. These factors should be considered when interpreting and designing trials in HER2-positive disease.
Trial registration: ClinicalTrials.gov NCT00770809.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Figures
Fig 1.
CONSORT diagram. pCR, pathologic complete response; QC, quality control; Rx, treatment; TH, paclitaxel plus trastuzumab; THL, paclitaxel, trastuzumab, and lapatinib; TL, paclitaxel plus lapatinib.
Fig 2.
Pathologic complete response (pCR) rates in breast by treatment arm, stratified by hormone receptor status. Error bars represent 95% confidence limits. TH, paclitaxel plus trastuzumab; THL, paclitaxel, trastuzumab, and lapatinib; TL, paclitaxel plus lapatinib.
Fig 3.
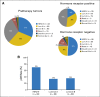
Treatment implications of molecular heterogeneity of human epidermal growth factor receptor 2 (HER2) –positive breast cancer. (A) Intrinsic subtype overall and by hormone receptor status among study population of clinically HER2-positive tumors, demonstrating that intrinsic subtype differed between hormone receptor–negative and –positive tumors (P < .001). (B) Pathologic complete response (pCR) rates by intrinsic subtype, demonstrating significant variation by intrinsic subtype (P < .001). Uncommon subtypes not shown. HER2-E, HER2 enriched; Lum, luminal.
Fig A1.
Expression of target genes HER2 (ERBB2), estrogen receptor (ESR1), and proliferation gene Ki67 (MKI67) with treatment among 55 tumors with paired pretherapy and residual disease samples, demonstrating significant decrease in human epidermal growth factor receptor 2 and proliferation gene expression, but no change in estrogen receptor expression. Normal breast gene expression is provided to illustrate baseline expression patterns.
Comment in
- Looking Deep Into the Heterogeneity of Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Can We Understand It Better?
Loi S, Savas P. Loi S, et al. J Clin Oncol. 2016 Feb 20;34(6):521-3. doi: 10.1200/JCO.2015.64.7495. Epub 2016 Jan 11. J Clin Oncol. 2016. PMID: 26755511 No abstract available.
References
- Blackwell KL, Burstein HJ, Storniolo AM, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2–positive metastatic breast cancer: Final results from the EGF104900 study. J Clin Oncol. 2012;30:2585–2592. - PubMed
- Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012;366:2438–2441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA33601/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- U10 CA181009/CA/NCI NIH HHS/United States
- UG1 CA233178/CA/NCI NIH HHS/United States
- 1U10CA180801/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA033601/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U10 CA180801/CA/NCI NIH HHS/United States
- P50 CA058223/CA/NCI NIH HHS/United States
- P50-CA58223/CA/NCI NIH HHS/United States
- U10CA180882/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U10 CA180791/CA/NCI NIH HHS/United States
- 1U10CA180838/CA/NCI NIH HHS/United States
- U10 CA180818/CA/NCI NIH HHS/United States
- 1U10CA180867/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- U10CA180821/CA/NCI NIH HHS/United States
- 1U10CA180791/CA/NCI NIH HHS/United States
- U10 CA180867/CA/NCI NIH HHS/United States
- U10 CA180838/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous