Ascorbic acid and ascorbate-2-phosphate decrease HIF activity and malignant properties of human melanoma cells - PubMed (original) (raw)
Ascorbic acid and ascorbate-2-phosphate decrease HIF activity and malignant properties of human melanoma cells
Sarah L Miles et al. BMC Cancer. 2015.
Abstract
Background: Hypoxia inducible factor-1 alpha (HIF-1α) is thought to play a role in melanoma carcinogenesis. Posttranslational regulation of HIF-1α is dependent on Prolyl hydroxylase (PHD 1-3) and Factor Inhibiting HIF (FIH) hydroxylase enzymes, which require ascorbic acid as a co-factor for optimal function. Depleted intra-tumoral ascorbic acid may thus play a role in the loss of HIF-1α regulation in melanoma. These studies assess the ability of ascorbic acid to reduce HIF-1α protein and transcriptional activity in metastatic melanoma and reduce its invasive potential.
Methods: HIF-1α protein was evaluated by western blot, while transcriptional activity was measured by HIF-1 HRE-luciferase reporter gene activity. Melanoma cells were treated with ascorbic acid (AA) and ascorbate 2-phosphate (A2P) to assess their ability to reduce HIF-1α accumulation and activity. siRNA was used to deplete cellular PHD2 in order to evaluate this effect on AA's ability to lower HIF-1α levels. A2P's effect on invasive activity was measured by the Matrigel invasion assay. Data was analyzed by One-way ANOVA with Tukey's multiple comparisons test, or Student-T test as appropriate, with p < .05 considered significant.
Results: Supplementation with both AA and A2P antagonized normoxic as well as cobalt chloride- and PHD inhibitor ethyl 3, 4-dihydroxybenzoate induced HIF-1α protein stabilization and transcriptional activity. Knockdown of the PHD2 isoform with siRNA did not impede the ability of AA to reduce normoxic HIF-1α protein. Additionally, reducing HIF-1α levels with A2P resulted in a significant reduction in the ability of the melanoma cells to invade through Matrigel.
Conclusion: These studies suggest a positive role for AA in regulating HIF-1α in melanoma by demonstrating that supplementation with either AA, or its oxidation-resistant analog A2P, effectively reduces HIF-1α protein and transcriptional activity in metastatic melanoma cells. Our data, while supporting the function of AA as a necessary cofactor for PHD and likely FIH activity, also suggests a potential non-PHD/FIH role for AA in HIF-1α regulation by its continued ability to reduce HIF-1α in the presence of PHD inhibition. The use of the oxidation-resistant AA analog, A2P, to reduce the ability of HIF-1α to promote malignant progression in melanoma cells and enhance their response to therapy warrants further investigation.
Figures
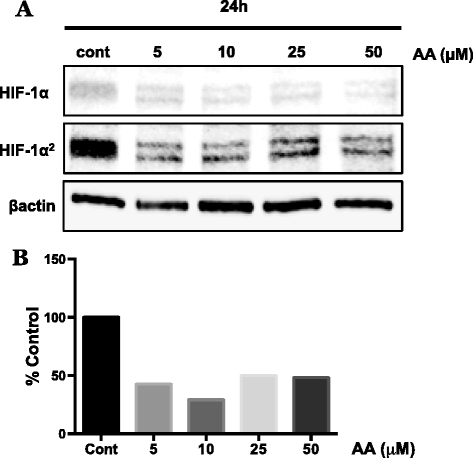
Fig. 1
Effect of ascorbic acid on HIF-1α stabilization in WM1366 radial growth phase melanoma cells. WM1366 cells were treated for 24 h with ascorbic acid (AA; 5–50 μM) under standard normoxic culture conditions. a Western blot analysis of isolated nuclear extracts reveals considerable reduction in the amount of stabilized HIF-1α protein following treatment with AA. b Densitometry analysis demonstrates the ability of AA supplementation at physiologically achievable concentrations to reduce the normoxic overexpression of HIF-1α by approximately 50-60 % in these cells. Protein expression was normalized to β-actin
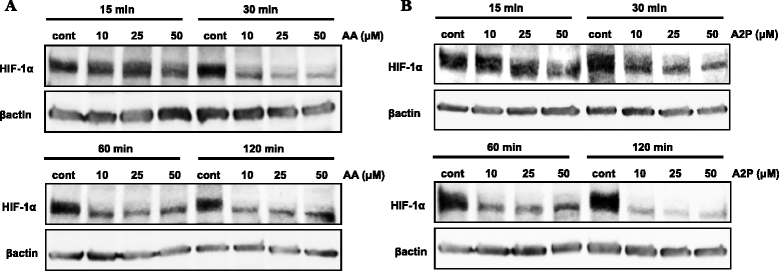
Fig. 2
Effect of ascorbic acid and ascorbate 2-phosphate on HIF-1α stabilization in WM9 metastatic melanoma cells. WM9 metastatic melanoma cells were treated with increasing concentrations (10, 25 or 50 μM) of ascorbic acid (AA), or the non-oxidizable analog ascorbate 2-phosphate (A2P) for 15–120 min under standard normoxic culture conditions. Western blot analysis of isolated nuclear fractions reveals that both (a) AA and (b) A2P cause nearly 50 % reduction of normoxic stabilized HIF-1α in these cells as early as 30 min following treatment. Treatment with A2P provided nearly complete reduction in stabilized HIF-1α by 120 min. Protein expression was normalized to β-actin. All treatments were repeated a minimum of 2 additional times with similar results
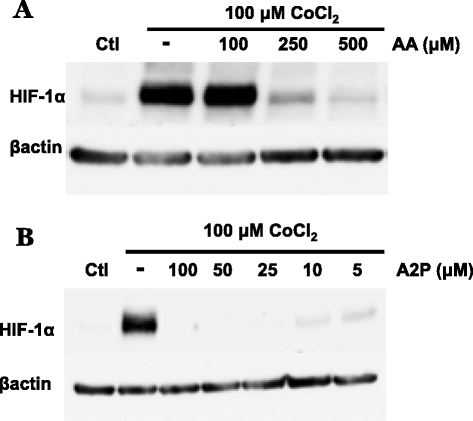
Fig. 3
Effect of AA and A2P on cobalt chloride induced HIF-1α protein accumulation in metastatic melanoma. WM9 metastatic melanoma cells were treated for 24 h with the hypoxia mimetic cobalt chloride (100 μM) in the presence or absence of AA and A2P and nuclear extracts analyzed by western blot. a The addition of AA (100–500 μM) reveals that 100 μM AA is unable to reduce CoCl2 induced HIF-1α accumulation. Higher concentrations of AA (250 and 500 μM) are necessary to reduce induced levels of HIF-1α b, while cells treated with A2P (5.0-100 μM) show that A2P efficiently reduces CoCl2 induced accumulation of HIF-1α at concentrations as low as 5 μM. Protein expression was normalized to β-actin. All treatments were repeated a minimum of 2 additional times with similar results
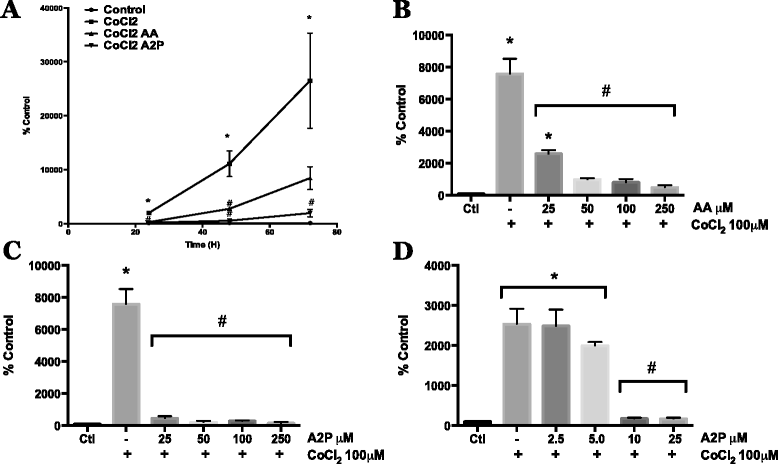
Fig. 4
Effect of AA and A2P on HIF-1α transcriptional activity in metastatic melanoma. WM9 metastatic melanoma cells were transiently transfected with an HIF-1 HRE-luciferase reporter vector. a Transfected WM9 cells were treated with 100 μM CoCl2 with or without AA (100 μM) or A2P (100 μM) Cells were collected and HIF-1 transcriptional activity was measured by luciferase assay at 24, 48, and 72 h. Both AA and A2P significantly reduced HIF-1 transcriptional activity at 24 and 48 h, at 72 h, A2P significantly reduced CoCl2 induced activity while AA began to show reduced efficacy by 72 h. b Dose dependent inhibition of CoCl2 induced HIF-1 reporter activity using 25, 50, 100 and 250 μM AA. AA significantly reduced CoCl2 induced HIF-1 activity at all concentrations, with 25 μM AA beginning to show reduced efficacy. c Dose dependent inhibition of CoCl2 induced HIF-1 reporter activity by 25, 50, 100 and 250 μM A2P. All concentrations of A2P significantly reduced CoCl2 induced HIF-1 reporter gene activity. For this reason, lower doses of A2P were then tested. d Low dose titration of A2P dependent inhibition of CoCl2 induced HIF-1 reporter activity using 2.5, 5.0, 10 and 25 μM A2P. A2P demonstrated close to maximum inhibition of HIF-1 activity at concentrations as low as 10 μM, with 5 and 2.5 μM demonstrating little to no inhibition of activity. All HRE-luciferase activity was normalized to β-galactosidase activity. Data are represented as mean ± SEM of a minimum of n = 3, analyzed by One-way ANOVA followed by Tukey’s multiple comparisons test; * denotes significant difference from control, p < 0.0001, # denotes significant difference from CoCl2 treatment alone, p < 0.003-0.0001
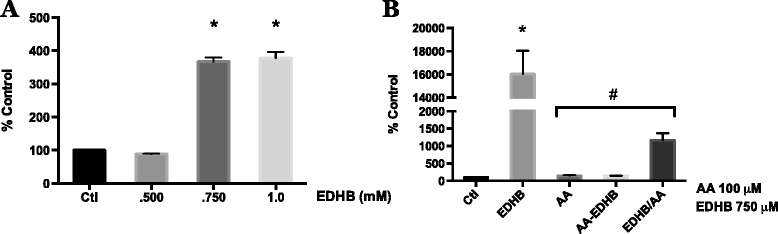
Fig. 5
Effect of ascorbic acid on EDHB induced HIF-1 transcriptional activity in melanoma cells. WM9 metastatic melanoma cells were transiently transfected with an HIF-1 HRE-luciferase reporter vector. a Transfected cells were treated for 24 h with 0.5, 0.75 and 1.0 mM EDHB. Induction of HIF-1 transcriptional activity was measured by luciferase assay. EDHB at 0.75 mM was found to be the lowest dose capable of generating near-maximal induction of HIF-1 transcriptional activity and was thus chosen for subsequent experiments. b Cells were treated for 24 h with 0.75 mM EDHB alone (EDHB), 100 μM AA alone (AA), pretreated with 100 μM AA for 4 h prior to addition of EDHB (AA-EDHB), or treated with100 μM AA and EDHB concurrently (EDHB/AA). AA effectively reduces EDHB induced HIF-1 transcriptional activity; with AA pretreatment showing increased efficacy at inhibiting EDHB induced HIF-1 activity vs. concomitant treatment. All HRE-luciferase activity was normalized to β-galactosidase activity. Data are represented as mean ± SEM of n = 3, analyzed by One-way ANOVA followed by Tukey’s multiple comparisons test; * denotes significant difference from control, p < 0.0001, # denotes significant difference from EDHB treatment alone, p < 0.0001
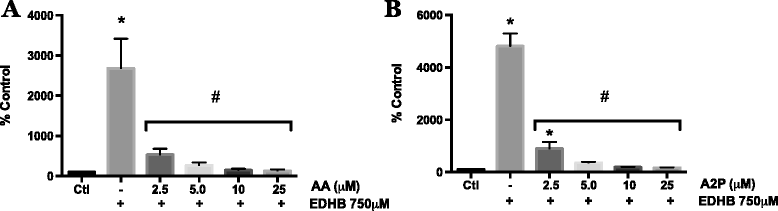
Fig. 6
Effect of AA and A2P on EDHB induced HIF-1 transcriptional activity in melanoma cells. WM9 metastatic melanoma cells were transiently transfected with an HIF-1 HRE-luciferase reporter vector. Transfected cells were treated for 24 h with 750 μM EDHB in the presence of a 2.5, 5.0, 10 or 25 μM AA or b 2.5, 5.0, 10 or 25 μM A2P. HIF-1 transcriptional activity was measured by luciferase assay. Data are presented as the mean ± SEM of n = 3, analyzed by One-way ANOVA followed by Tukey’s multiple comparisons test; * denotes significant difference from control, p < 0.0001, # denotes significant difference from EDHB treatment alone, p < 0.0001
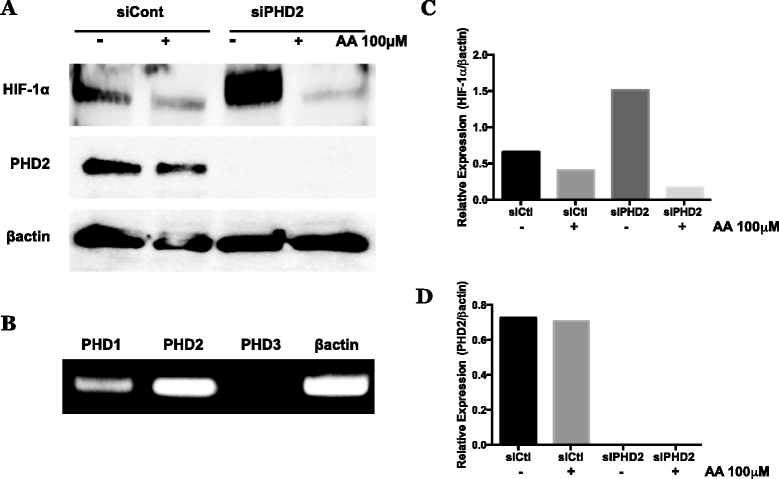
Fig. 7
Effect of PHD2 knockdown on reduction of normoxic HIF-1α protein by AA in metastatic melanoma. WM9 metastatic melanoma cells were transfected using non-targeting control siRNA or siGENOME SMARTpool siRNA against PHD2. a siRNA transfected cells were treated for 24 h with or without 100 μM AA under standard normoxic culture conditions. HIF-1α and PHD2 were analyzed by western blot, and normalized to β-actin. Knockdown of PHD2 caused an increase in stabilized HIF-1α protein, however, does not result in loss of effectiveness of AA to reduce accumulated HIF-1α. b qPCR analysis of PHD1, 2, and 3 isoforms in untreated WM9 metastatic melanoma cells, normalized to β-actin expression. PHD2 appears is the prevalent isoform, however the presences of PHD1 may contribute to the retained activity of AA following PHD2 selective knockdown. c, d Densitometry analysis of HIF-1α and PHD2 expression following PHD2 knockdown and AA treatment. siRNA experiments were repeated a minimum of 2 additional times with similar results
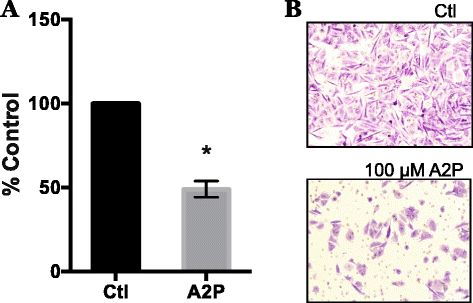
Fig. 8
Effect of A2P treatment on invasive potential of metastatic melanoma cells. WM9 metastatic melanoma cells were maintained in 100 μM A2P for 5 days under standard normoxic culture conditions. Cells were seeded into Matrigel chambers and assayed for invasion after 24 h. a Matrigel invasion assay was completed as described in Methods and Materials. Cells grown in the presence of A2P demonstrated a 50 % reduction in invasion. b Representative photographs of Matrigel invasion chambers. Data are represented as mean ± SEM of n = 3, analyzed by Student paired _T_-test; * denotes significant difference from control, p < 0.0087
Similar articles
- Silencing HIF-1α induces TET2 expression and augments ascorbic acid induced 5-hydroxymethylation of DNA in human metastatic melanoma cells.
Fischer AP, Miles SL. Fischer AP, et al. Biochem Biophys Res Commun. 2017 Aug 19;490(2):176-181. doi: 10.1016/j.bbrc.2017.06.017. Epub 2017 Jun 8. Biochem Biophys Res Commun. 2017. PMID: 28601635 - Expression and function of hypoxia inducible factor-1 alpha in human melanoma under non-hypoxic conditions.
Mills CN, Joshi SS, Niles RM. Mills CN, et al. Mol Cancer. 2009 Nov 17;8:104. doi: 10.1186/1476-4598-8-104. Mol Cancer. 2009. PMID: 19919690 Free PMC article. - Hypoxic microenvironment as a cradle for melanoma development and progression.
Michaylira CZ, Nakagawa H. Michaylira CZ, et al. Cancer Biol Ther. 2006 May;5(5):476-9. doi: 10.4161/cbt.5.5.2749. Epub 2006 May 26. Cancer Biol Ther. 2006. PMID: 16627974 Review. - NF-κB mediated regulation of tumor cell proliferation in hypoxic microenvironment.
Rastogi S, Aldosary S, Saeedan AS, Ansari MN, Singh M, Kaithwas G. Rastogi S, et al. Front Pharmacol. 2023 Feb 20;14:1108915. doi: 10.3389/fphar.2023.1108915. eCollection 2023. Front Pharmacol. 2023. PMID: 36891273 Free PMC article. Review.
Cited by
- Redox-active vitamin C suppresses human osteosarcoma growth by triggering intracellular ROS-iron-calcium signaling crosstalk and mitochondrial dysfunction.
Vaishampayan P, Lee Y. Vaishampayan P, et al. Redox Biol. 2024 Sep;75:103288. doi: 10.1016/j.redox.2024.103288. Epub 2024 Jul 26. Redox Biol. 2024. PMID: 39083898 Free PMC article. - Role of Vitamin C in Skin Diseases.
Wang K, Jiang H, Li W, Qiang M, Dong T, Li H. Wang K, et al. Front Physiol. 2018 Jul 4;9:819. doi: 10.3389/fphys.2018.00819. eCollection 2018. Front Physiol. 2018. PMID: 30022952 Free PMC article. Review. - Ascorbyl palmitate-incorporated paclitaxel-loaded composite nanoparticles for synergistic anti-tumoral therapy.
Zhou M, Li X, Li Y, Yao Q, Ming Y, Li Z, Lu L, Shi S. Zhou M, et al. Drug Deliv. 2017 Nov;24(1):1230-1242. doi: 10.1080/10717544.2017.1370619. Drug Deliv. 2017. PMID: 28856937 Free PMC article. - Insights into Differentiation of Melanocytes from Human Stem Cells and Their Relevance for Melanoma Treatment.
Mirea MA, Eckensperger S, Hengstschläger M, Mikula M. Mirea MA, et al. Cancers (Basel). 2020 Sep 3;12(9):2508. doi: 10.3390/cancers12092508. Cancers (Basel). 2020. PMID: 32899370 Free PMC article. Review. - Ascorbic acid induced TET2 enzyme activation enhances cancer immunotherapy efficacy in renal cell carcinoma.
Peng D, He A, He S, Ge G, Wang S, Ci W, Li X, Xia D, Zhou L. Peng D, et al. Int J Biol Sci. 2022 Jan 1;18(3):995-1007. doi: 10.7150/ijbs.67329. eCollection 2022. Int J Biol Sci. 2022. PMID: 35173532 Free PMC article.
References
- Serrone L, Zeuli M, Sega FM, Cognetti F. Dacarbazine-based chemotherapy for metastatic melanoma: thirty-year experience overview. J Exp Clin Cancer Res. 2000;19(1):21–34. - PubMed
- Berrocal A, Cabanas L, Espinosa E, Fernandez-de-Misa R, Martin-Algarra S, Martinez-Cedres JC et al. Melanoma: Diagnosis, Staging, and Treatment. Consensus group recommendations. Advances in therapy. 2014. doi:10.1007/s12325-014-0148-2 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical