5' UTR m(6)A Promotes Cap-Independent Translation - PubMed (original) (raw)
5' UTR m(6)A Promotes Cap-Independent Translation
Kate D Meyer et al. Cell. 2015.
Abstract
Protein translation typically begins with the recruitment of the 43S ribosomal complex to the 5' cap of mRNAs by a cap-binding complex. However, some transcripts are translated in a cap-independent manner through poorly understood mechanisms. Here, we show that mRNAs containing N(6)-methyladenosine (m(6)A) in their 5' UTR can be translated in a cap-independent manner. A single 5' UTR m(6)A directly binds eukaryotic initiation factor 3 (eIF3), which is sufficient to recruit the 43S complex to initiate translation in the absence of the cap-binding factor eIF4E. Inhibition of adenosine methylation selectively reduces translation of mRNAs containing 5'UTR m(6)A. Additionally, increased m(6)A levels in the Hsp70 mRNA regulate its cap-independent translation following heat shock. Notably, we find that diverse cellular stresses induce a transcriptome-wide redistribution of m(6)A, resulting in increased numbers of mRNAs with 5' UTR m(6)A. These data show that 5' UTR m(6)A bypasses 5' cap-binding proteins to promote translation under stresses.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
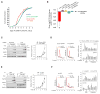
Figure 1. 5′UTR m6A Enables Ribosome Binding to mRNA in the Absence of Cap-Binding Proteins
(A) 5′ UTR methylation permits 48S initiation complex formation in the absence of the group 4 eIFs. In vitro transcribed, capped mRNAs encoding a MVHC tetrapeptide and containing either A or m6A were incubated with purified mammalian translation initiation components. Subsequent toeprinting analysis using a radiolabeled primer then revealed whether 48S initiation complexes were formed. Positions of the initiation codon, full-length cDNA, and the 48S complex are shown on the sides of the panel. Lanes C/T/A/G depict the corresponding DNA sequence. When unmethylated mRNA is used (lanes 1–5), 48S complexes are only formed when the cap-binding complex eIF4F is present (lanes 2 and 3). When eIF4F is absent, 48S complex formation on unmethylated mRNA is impaired (lanes 4 and 5). However, when mRNA with m6A in the 5′ UTR is used, 48S complex formation is observed even in the absence of eIF4F (lanes 9 and 10; compare to lanes 7 and 8 where eIF4F is present). (B) eIFs1, 1A, and 3 are required for efficient m6A-induced cap-independent 48S complex formation. Toeprinting assays were performed as in (A) using A- or m6A-containing mRNAs and in the presence of various translation initiation components as indicated. m6A-containing mRNA exhibits robust 48S complex assembly in the absence of eIF4F, whereas A-containing mRNA does not (compare lanes 1 and 7). Efficient m6A-mediated 48S complex assembly is also dependent on the presence of eIFs1 and 1A, which is consistent with the known roles of these proteins in promoting scanning and AUG recognition (compare lanes 1 with lanes 2, 4, and 5). Removal of eIF3 also abolishes 48S complex assembly on m6A-containing mRNA (compare lanes 1 and 2), indicating that eIF3 is required for m6A-mediated 48S complex formation. Addition of 60S subunits, eIF5, eIF5B, eEF1H, eEF2, and aa-tRNAs resulted in the appearance of toeprints corresponding to pre-termination complexes at the stop codon, indicating that m6A-recruited 48S complexes are fully functional (lane 6). (C) Omission of eIF2 from toeprinting assays results in the absence of 48S complexes (compare lanes 2 and 3), indicating that eIF2 is required for 48S complex assembly on m6A-containing mRNA.
Figure 2. m6A within the 5′ UTR Enables Cap-Independent Translation of mRNA
(A) 5′ UTR m6A permits mRNA translation without the need for the cap-binding protein eIF4E. In vitro translation was performed using a HeLa cell extract mixed with luciferase-encoding, capped mRNA containing either A or m6A. Protein production was measured by quantifying luciferase activity. Cap-dependent translation is observed from both methylated and unmethylated mRNAs in the presence of eIF4E. However, when eIF4E is absent, only the m6A-containing mRNA is translated (n = 4; mean ± SD; ***p < 0.0001). (B) Presence of a 5′ cap analog is unable to abolish m6A-induced mRNA translation. Luciferase mRNAs were translated as in (A). 1 mM free cap analog (m7GpppG) was added to sequester cap-binding proteins. Addition of m7GpppG abolishes cap-dependent translation of unmethylated mRNA (left) but is unable to abolish the cap-independent translation induced by m6A (right). Levels of luciferase activity are shown relative to capped mRNA + 10 pmole eIF4E (n = 3; mean ± SD; *p < 0.01, **p < 0.001). (C) In vitro translation was performed using luciferase-encoding mRNA containing A or 50% m6A and with or without a 5′cap as indicated. While unmethylated, capped mRNA + 10 pmole eIF4E is robustly translated, the unmethylated, uncapped mRNA fails to be translated. However, m6A-containing mRNA is efficiently translated even when no 5′ cap is present (n = 3; mean ± SD; *p < 0.01). (D) m6A residues in the coding sequence do not induce cap-independent translation. Uncapped, luciferase-encoding mRNAs containing either the natural β-globin 5′ UTR or a modified β-globin 5′ UTR containing either zero, one, or three A residues as indicated were used for in vitro translation assays. Translation of m6A-containing mRNA with zero A residues in the 5′ UTR was markedly diminished, indicating that coding sequence m6A residues are unable to induce cap-independent translation. However, when a single m6A was added to the 5′ UTR, the transcripts were robustly translated. Methylated 5′ UTRs with a single A near the 5′ end, the middle (mid), or near the 3′ end all showed similar levels of translation (n = 3; mean ± SD; **p < 0.001, ***p < 0.0001, ****p < 0.00001). Schematic shows the distribution of A residues within each β-globin 5′ UTR variant (the unmodified β-globin 5′ UTR contains 17 A residues). (E) mRNA with a single m6A within the 5′ UTR and no m6As in the remainder of the transcript induces cap-independent translation. Uncapped, luciferase-encoding mRNAs, which contained either a single adenosine 5′-monophosphate (AMP) or _N_6-methyladenosine 5′-monophosphate (m6AMP) at the 5′ end, were used for in vitro translation. Only the m6A-containing mRNA was translated, demonstrating that a single 5′ end m6A residue is capable of inducing cap-independent translation (n = 3; mean ± SD; **p < 0.001). The reduced translation efficiency of this mRNA compared to mRNAs with internally methylated 5′ UTRs is likely due to inefficient incorporation of m6A residues at the 5′ end by T7 RNA polymerase. See also Figure S1.
Figure 3. m6A-Mediated Translation Occurs through a 5′ End-Dependent Mechanism
(A) Toeprinting assays were performed using a capped, m6A-containing mRNA containing the β-globin 5′ UTR sequence, which was modified to include two AUG initiation codons (“AUG1” and “AUG2” in the schematic). The majority of 48S complexes were assembled at AUG1, with negligible levels of 48S complexes detected at AUG2. (B) Uncapped, A-, or m6A-containing mRNAs encoding GFP were used for in vitro translation. The mRNA contains two near-kozak start codons: AUG 1 encodes the full-length GFP protein, and internally localized AUG2 encodes an in-frame truncated (~17 kDa) protein comprising the C-terminal portion of GFP. Full-length and truncated GFP protein levels (sizes indicated by arrows) were measured by western blot. m6A primarily promotes translation of the full-length protein and fails to induce internal entry-mediated translation from AUG2. Levels of the ribosomal protein RPS6 are shown as a loading control. (C) Quantification of full-length GFP protein levels in (B) shows increased protein expression of methylated mRNA versus unmethylated mRNA (n = 3; mean ± SD; **p < 0.001). (D) The presence of a stable hairpin at the beginning of the 5′ UTR to block 5′ end entry severely attenuates m6A-mediated translation (n = 3; mean ± SD; *p < 0.01). See also Figure S1.
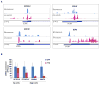
Figure 4. The 43S Complex Component eIF3 Binds m6A
(A) Indicated proteins/protein complexes were incubated with radiolabeled A- or m6A-containing RNA probes and crosslinked. Unbound RNAs were then removed with RNase I, proteins were separated by SDS-PAGE, and radioactively-labeled RNAs were detected. eIF1, eIF1A, eIF2, and the 40S ribosomal subunit show no preferential crosslinking to methylated RNA. However, eIF3 preparations exhibit strong crosslinking to methylated RNA at bands around 60 kD, 80 kD, and 110–160 kD, which correspond to multiple subunits of the eIF3 complex as indicated. (B) Crosslinking assays were performed as in (A) using the HeLa cell extracts utilized in in vitro translation assays. The eIF3 complex was immunoprecipitated using antibodies against eIF3a or eIF3b, and proteins containing crosslinked RNA were detected. Both eIF3 antibodies precipitated proteins that preferentially crosslinked to m6A RNA. Immunoprecipitation using rabbit and mouse IgG control antibodies are shown as negative controls. Western blotting for the indicated proteins indicates their enrichment following immunoprecipitation (bottom). The input lanes throughout have 25% of the material loaded for the IP lanes. See also Figures S2 and S3.
Figure 5. eIF3 Binding Sites within Cellular mRNAs Localize to Sites of m6A Residues within the 5′ UTR
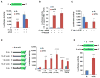
(A) Shown are read clusters from both eIF3 PAR-iCLIP (light blue) and single-nucleotide-resolution m6A mapping (Linder et al., 2015) (miCLIP; red) for four representative mRNAs (EIF4A3, H3F3C, SQLE, and IER5). eIF3a PAR-iCLIP read clusters exhibit highly specific overlap with m6A mapping clusters at internal positions within 5′ UTRs. This co-localization is specific to 5′ UTRs, as mRNAs that contain multiple m6A residues in the CDS or 3′ UTR fail to show eIF3a binding at these sites (exemplified by IER5). Red asterisks indicate the location of individual m6A sites identified at single-nucleotide resolution. (B) eIF3 binds to the 5′ UTR of cellular mRNAs in an m6A-dependent manner. HEK293 cells were transfected with GFP- or Fto-overexpression plasmids, and eIF3 immunoprecipitation was performed to isolate eIF3-bound mRNAs. Bound mRNAs were quantified by RT-qPCR using 5′ UTR-specific primers. 5′ UTRs of mRNAs that contain high levels of m6A exhibited reduced binding to eIF3 after overexpression of Fto. 5′ UTRs that do not contain m6A exhibited no change in eIF3 binding following Fto overexpression (n = 3; mean ± SEM). See also Figures S4 and S5.
Figure 6. m6A Mediates Stress-Induced Translation of Hsp70
(A) Depletion of the m6A methyltransferase, METTL3, decreases the TE of mRNAs with 5′ UTR m6A. Ribosome profiling data from HeLa cells expressing METTL3 or control siRNAs (Wang et al., 2015) were used to determine changes in TE for various classes of mRNAs defined by single-nucleotide-resolution m6A mapping. Compared to nonmethylated mRNAs (blue), transcripts with m6A residues in the coding sequence (CDS) or 3′ UTR (green) exhibit only a marginal decrease in TE. However, mRNAs containing m6A within the 5′ UTR (red) show a large reduction in TE. p values were calculated using the Mann-Whitney test. (B) TEs of various classes of m6A-containing mRNAs were analyzed using ribosome profiling datasets from HeLa cells as described in (A). Shown are the mean fold changes in TE (siMETTL3/siControl) for mRNAs with m6A residues only in the 5′ UTR (red), within the 3′ UTR (purple), within 50 nt of the stop codon (yellow), within the CDS and/or 3′ UTR (green), or in all mRNAs (blue), as defined by single-nucleotide-resolution m6A mapping. mRNAs with 5′ UTR m6A residues exhibit a dramatic reduction in TE after METTL3 depletion, whereas transcripts with m6As in other regions fail to show this effect. All mean fold change TE values were computed after background subtraction of the mean fold change computed from all nonmethylated control mRNAs, as indicated by the arrow (mean ± SEM; *p < 0.05). (C) Fto knockdown increases heat-shock-induced translation of Hsp70. MEF cells stably expressing either Fto shRNA or scramble shRNA were subjected to heat shock stress. Cell lysates were collected at various times post-heat shock (“Post HS”) and then used for western blot analysis with the indicated antibodies. Fto knockdown increased the levels of stress-induced Hsp70 protein compared to control shRNA (“S exp” = short exposure; “L exp” = long exposure). Levels of Hsp25, another heat shock-induced protein, were unaffected by Fto knockdown. Right panel shows quantification of Hsp70 levels normalized to β-actin (n = 3; mean ± SEM; **p < 0.1). (D) MEFs stably expressing control or Fto shRNA were subjected to heat shock stress as in (C). Polysome fractions were separated using sucrose gradient fractionation (left panels) followed by RT-qPCR for Hsp70 (top right panel) and Gapdh (bottom right panel) in each fraction. Hsp70 levels are increased in polysome fractions following Fto knockdown, whereas the distribution of Gapdh is unchanged (n = 3; mean ± SEM; Hsp70: p = 0.0007, two-way ANOVA; Gapdh: p = 0.3722, two-way ANOVA considering the entire range of time points). (E) MEF cells were infected with either GFP or Fto lentivirus and subjected to heat shock stress. Cell lysates were collected at various times post-heat shock and then used for western blot analysis with the indicated antibodies. Fto overexpression decreased the levels of heat-shock-induced Hsp70 protein compared to GFP overexpression. Levels of Hsp25 were unaffected by Fto overexpression. Right panel shows quantification of Hsp70 levels normalized to β-actin (n = 3; Mean ± SEM; *p < 0.5). (F) MEFs with or without Fto overexpression were subjected to heat shock stress as in (E). Polysome fractions were separated using sucrose gradient fractionation (left panels) followed by RT-qPCR of Hsp70 (top right panel) and Gapdh (bottom right panel) in each fraction. Hsp70 levels are decreased in polysome fractions following Fto overexpression, whereas the distribution of Gapdh is unchanged (n = 3; mean ± SEM; Hsp70: p < 0.0001, two-way ANOVA; Gapdh: p = 0.1910, two-way ANOVA considering the entire range of time points). See also Figures S5 and S6 and Table S1.
Similar articles
- Dynamic m(6)A mRNA methylation directs translational control of heat shock response.
Zhou J, Wan J, Gao X, Zhang X, Jaffrey SR, Qian SB. Zhou J, et al. Nature. 2015 Oct 22;526(7574):591-4. doi: 10.1038/nature15377. Epub 2015 Oct 12. Nature. 2015. PMID: 26458103 Free PMC article. - Internal ribosome entry site drives cap-independent translation of reaper and heat shock protein 70 mRNAs in Drosophila embryos.
Hernández G, Vázquez-Pianzola P, Sierra JM, Rivera-Pomar R. Hernández G, et al. RNA. 2004 Nov;10(11):1783-97. doi: 10.1261/rna.7154104. RNA. 2004. PMID: 15496524 Free PMC article. - Eukaryotic initiation factor (eIF) 3 mediates Barley Yellow Dwarf Viral mRNA 3'-5' UTR interactions and 40S ribosomal subunit binding to facilitate cap-independent translation.
Bhardwaj U, Powell P, Goss DJ. Bhardwaj U, et al. Nucleic Acids Res. 2019 Jul 9;47(12):6225-6235. doi: 10.1093/nar/gkz448. Nucleic Acids Res. 2019. PMID: 31114905 Free PMC article. - More than just scanning: the importance of cap-independent mRNA translation initiation for cellular stress response and cancer.
Lacerda R, Menezes J, Romão L. Lacerda R, et al. Cell Mol Life Sci. 2017 May;74(9):1659-1680. doi: 10.1007/s00018-016-2428-2. Epub 2016 Dec 2. Cell Mol Life Sci. 2017. PMID: 27913822 Free PMC article. Review. - Cap-dependent, scanning-free translation initiation mechanisms.
Haimov O, Sinvani H, Dikstein R. Haimov O, et al. Biochim Biophys Acta. 2015 Nov;1849(11):1313-8. doi: 10.1016/j.bbagrm.2015.09.006. Epub 2015 Sep 14. Biochim Biophys Acta. 2015. PMID: 26381322 Review.
Cited by
- Global Co-regulatory Cross Talk Between m6A and m5C RNA Methylation Systems Coordinate Cellular Responses and Brain Disease Pathways.
Orji OC, Stones J, Rajani S, Markus R, Öz MD, Knight HM. Orji OC, et al. Mol Neurobiol. 2024 Nov 5. doi: 10.1007/s12035-024-04555-0. Online ahead of print. Mol Neurobiol. 2024. PMID: 39499421 - CircRNAs: biogenesis, functions, and role in drug-resistant Tumours.
Ma S, Kong S, Wang F, Ju S. Ma S, et al. Mol Cancer. 2020 Aug 5;19(1):119. doi: 10.1186/s12943-020-01231-4. Mol Cancer. 2020. PMID: 32758239 Free PMC article. Review. - m6A Modifications Play Crucial Roles in Glial Cell Development and Brain Tumorigenesis.
Wang J, Sha Y, Sun T. Wang J, et al. Front Oncol. 2021 Feb 24;11:611660. doi: 10.3389/fonc.2021.611660. eCollection 2021. Front Oncol. 2021. PMID: 33718165 Free PMC article. Review. - FMRP phosphorylation modulates neuronal translation through YTHDF1.
Zou Z, Wei J, Chen Y, Kang Y, Shi H, Yang F, Shi Z, Chen S, Zhou Y, Sepich-Poore C, Zhuang X, Zhou X, Jiang H, Wen Z, Jin P, Luo C, He C. Zou Z, et al. Mol Cell. 2023 Dec 7;83(23):4304-4317.e8. doi: 10.1016/j.molcel.2023.10.028. Epub 2023 Nov 9. Mol Cell. 2023. PMID: 37949069 Free PMC article. - m(6)A: Signaling for mRNA splicing.
Adhikari S, Xiao W, Zhao YL, Yang YG. Adhikari S, et al. RNA Biol. 2016 Sep;13(9):756-9. doi: 10.1080/15476286.2016.1201628. Epub 2016 Jun 28. RNA Biol. 2016. PMID: 27351695 Free PMC article. Review.
References
- Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. - PubMed
- Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999;68:913–963. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM059660/GM/NIGMS NIH HHS/United States
- K99MH104712/MH/NIMH NIH HHS/United States
- R01 CA186702/CA/NCI NIH HHS/United States
- R01 AG042400/AG/NIA NIH HHS/United States
- K99 MH104712/MH/NIMH NIH HHS/United States
- R01DA037755/DA/NIDA NIH HHS/United States
- GM59660/GM/NIGMS NIH HHS/United States
- AG042400/AG/NIA NIH HHS/United States
- R01 DA037755/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous