The Hippo pathway mediates inhibition of vascular smooth muscle cell proliferation by cAMP - PubMed (original) (raw)
The Hippo pathway mediates inhibition of vascular smooth muscle cell proliferation by cAMP
Tomomi E Kimura et al. J Mol Cell Cardiol. 2016 Jan.
Abstract
Aims: Inhibition of vascular smooth muscle cell (VSMC) proliferation by intracellular cAMP prevents excessive neointima formation and hence angioplasty restenosis and vein-graft failure. These protective effects are mediated via actin-cytoskeleton remodelling and subsequent regulation of gene expression by mechanisms that are incompletely understood. Here we investigated the role of components of the growth-regulatory Hippo pathway, specifically the transcription factor TEAD and its co-factors YAP and TAZ in VSMC.
Methods and results: Elevation of cAMP using forskolin, dibutyryl-cAMP or the physiological agonists, Cicaprost or adenosine, significantly increased phosphorylation and nuclear export YAP and TAZ and inhibited TEAD-luciferase report gene activity. Similar effects were obtained by inhibiting RhoA activity with C3-transferase, its downstream kinase, ROCK, with Y27632, or actin-polymerisation with Latrunculin-B. Conversely, expression of constitutively-active RhoA reversed the inhibitory effects of forskolin on TEAD-luciferase. Forskolin significantly inhibited the mRNA expression of the pro-mitogenic genes, CCN1, CTGF, c-MYC and TGFB2 and this was reversed by expression of constitutively-active YAP or TAZ phospho-mutants. Inhibition of YAP and TAZ function with RNAi or Verteporfin significantly reduced VSMC proliferation. Furthermore, the anti-mitogenic effects of forskolin were reversed by overexpression of constitutively-active YAP or TAZ.
Conclusion: Taken together, these data demonstrate that cAMP-induced actin-cytoskeleton remodelling inhibits YAP/TAZ-TEAD dependent expression of pro-mitogenic genes in VSMC. This mechanism contributes novel insight into the anti-mitogenic effects of cAMP in VSMC and suggests a new target for intervention.
Keywords: 3′-5′-Cyclic adenosine monophosphate; TAZ; TEAD; VSMC; YAP; cAMP.
Copyright © 2015 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
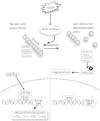
Graphical abstract
Fig. 1
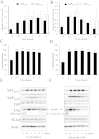
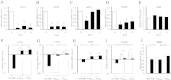
Elevated cAMP induces phosphorylation of YAP and TAZ in VSMC. VSMC were stimulated with 25 μM forskolin (A, C, E) or 500 μM db-cAMP (B, D, F) for the indicated times and total cell lysates analysed by Western blotting for phospho-YAP (S127 and S397; A, B, E, F) and total-TAZ phosphorylation (C–F). Western blots were quantified using densitometry (A–D). All experiments are at least n = 4. * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
Fig. 2
Elevated cAMP induces YAP/TAZ nuclear export and TAZ degradation. VSMC were stimulated with 25 μM forskolin for the indicated times. (A) Total cell lysates were analysed fot YAP and TAZ (n = 3). (B) Cytoplasmic and nuclear fractions were analysed for YAP, TAZ, Lamin A/C and GAPDH. *** indicates p < 0.001.
Fig. 3
Elevated cAMP inhibited TEAD-dependent transcription. VSMCs were transfected with either TEAD-luciferase plasmid (8xTEAD) or TnT-minimal promoter-luciferase plasmid (TnT-minP) that lacks TEAD elements. Cells were stimulated with 25 μM forskolin (A and C) or 500 μM db-cAMP (B) for the indicated times. Cell lysates were assayed for luciferase activity. (C) VSMCs were transfected with TEAD-luciferase plasmid together with active YAP (YAPS127A), active TAZ (TAZ4SA), or empty control vector. The cells ere stimulated with 25 μM forskolin for 8 h. All data is n = 3 * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
Fig. 4
Inhibition of RhoA/ROCK-mediated actin polymerisation underlies cAMP-mediated repression of YAP/TAZ and TEAD. VSMCs were treated with RhoA inhibitor (C3 Transferase; 2 μg/ml), ROCK inhibitor (Y-27,632; 10 μM) or actin-polymerisation inhibitor (Latrunculin B; 5 μg/ml) for 30 min and phosphorylation of YAP/TAZ was quantified Western blotting (A–C). VSMCs were transfected with TEAD-luciferase plasmid (8xTEAD-LUC) or TnT-minimal promoter-luciferase plasmid (TnT-minP) and treated with indicated RhoA/ROCK pathway inhibitors for 8 h (D). Cells infected with either active RhoA (RhoAG14V) or control adenovirus were stimulated with 25 μM forskolin for 8 h (E). All data is n = 3. * indicates p < 0.05, ** indicates p < 0.01. ## indicates p < 0.01 and ### indicates p < 0.001 on log transformed data.
Fig. 5
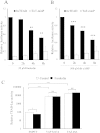
cAMP inhibits the expression YAP/TAZ-dependent pro-mitogenic genes. VSMCs were stimulated with 25 μM forskolin for the indicated times (A–F) and analysed by qRT-PCR for CCN1 (A; n = 4), CTGF (B; n = 4), cMYC (C; n = 8), TGFB2 (D; n = 8), 36B4 (E; n = 4). Cells were infected with control adenovirus, YAPS127A adenovirus or TAZ4SA adenovirus and stimulated with 25 μM forskolin (F–J). Expression of CCN1 (F; n = 4), CTGF (G; n = 4), cMYC (H; n = 4), TGFB2 (I; n = 4), 36B4 (J; n = 4) was analysed by qRT-PCR. * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
Fig. 6
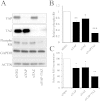
YAP and TAZ cooperate to regulate VSMC proliferation. VSMC were transfected with siRNA targeting YAP, TAZ or YAP plus TAZ. Total cell lysates were prepared 24 h post transfection and analysed by Western blotting for YAP, TAZ, phospho-Rb, GAPDH and ACTIN (A). Phospho-Rb levels were quantified by densitometry (B; n = 3). siRNA-transfected cells were labelled with BrdU between 24 and 36 h post transfection (C; n = 3). * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
Fig. 7
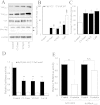
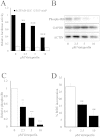
The YAP–TEAD inhibitor Veteporfin inhibits VSMC proliferation. VSMC were transfected with TEAD-luciferase reporter plasmid (8xTEAD) or a TnT-minimal promoter-luciferase plasmid (TnT-minP) and treated with the indicated concentrations of Verteporfin for 8 h (A; n = 3). VSMCs were treated with the indicated concentrations of Verteporfin for 18 h and total cell lysates analysed for phospho-Rb, GAPDH and ACTIN levels by Western blotting (B). Phospho-Rb Western blots were quantified by densitometry (C; n = 3). VSMCs were treated with Verteporfin for 18 h followed by BrdU labelling for 6 h (D; n = 3). * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
Fig. 8
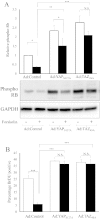
Constitutively active YAP and TAZ reverse the anti-mitogenic effect of forskolin in VSMC. VSMCs were infected with 3 × 107 pfu/ml of control adenovirus or adenovirus expressing constitutively active YAPS127A or TAZ4SA. Cells were stimulated with 25 μM forskolin for 18 h (A and B). Total cell lysates were analysed for phospho-Rb levels by Western blotting and densitometry (A; n = 3). Cells were labelled with BrDU for 6 h immediately after forskolin stimulations (B; n = 3). * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
Similar articles
- cAMP-induced actin cytoskeleton remodelling inhibits MKL1-dependent expression of the chemotactic and pro-proliferative factor, CCN1.
Duggirala A, Kimura TE, Sala-Newby GB, Johnson JL, Wu YJ, Newby AC, Bond M. Duggirala A, et al. J Mol Cell Cardiol. 2015 Feb;79:157-68. doi: 10.1016/j.yjmcc.2014.11.012. Epub 2014 Nov 18. J Mol Cell Cardiol. 2015. PMID: 25446180 Free PMC article. - Inhibition of Egr1 expression underlies the anti-mitogenic effects of cAMP in vascular smooth muscle cells.
Kimura TE, Duggirala A, Hindmarch CC, Hewer RC, Cui MZ, Newby AC, Bond M. Kimura TE, et al. J Mol Cell Cardiol. 2014 Jul;72(100):9-19. doi: 10.1016/j.yjmcc.2014.02.001. Epub 2014 Feb 15. J Mol Cell Cardiol. 2014. PMID: 24534707 Free PMC article. - Elevated cyclic-AMP represses expression of exchange protein activated by cAMP (EPAC1) by inhibiting YAP-TEAD activity and HDAC-mediated histone deacetylation.
Ebrahimighaei R, McNeill MC, Smith SA, Wray JP, Ford KL, Newby AC, Bond M. Ebrahimighaei R, et al. Biochim Biophys Acta Mol Cell Res. 2019 Oct;1866(10):1634-1649. doi: 10.1016/j.bbamcr.2019.06.013. Epub 2019 Jun 28. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 31255721 - Ending Restenosis: Inhibition of Vascular Smooth Muscle Cell Proliferation by cAMP.
Smith SA, Newby AC, Bond M. Smith SA, et al. Cells. 2019 Nov 16;8(11):1447. doi: 10.3390/cells8111447. Cells. 2019. PMID: 31744111 Free PMC article. Review. - Hippo pathway inhibition by blocking the YAP/TAZ-TEAD interface: a patent review.
Crawford JJ, Bronner SM, Zbieg JR. Crawford JJ, et al. Expert Opin Ther Pat. 2018 Dec;28(12):867-873. doi: 10.1080/13543776.2018.1549226. Epub 2018 Dec 2. Expert Opin Ther Pat. 2018. PMID: 30482112 Review.
Cited by
- Notch signaling regulates strain-mediated phenotypic switching of vascular smooth muscle cells.
Karakaya C, van Turnhout MC, Visser VL, Ristori T, Bouten CVC, Sahlgren CM, Loerakker S. Karakaya C, et al. Front Cell Dev Biol. 2022 Aug 12;10:910503. doi: 10.3389/fcell.2022.910503. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36036000 Free PMC article. - Combined role for YAP-TEAD and YAP-RUNX2 signalling in substrate-stiffness regulation of cardiac fibroblast proliferation.
Ebrahimighaei R, Sala-Newby GB, Hudson C, Kimura TE, Hathway T, Hawkins J, McNeill MC, Richardson R, Newby AC, Bond M. Ebrahimighaei R, et al. Biochim Biophys Acta Mol Cell Res. 2022 Nov;1869(11):119329. doi: 10.1016/j.bbamcr.2022.119329. Epub 2022 Jul 26. Biochim Biophys Acta Mol Cell Res. 2022. PMID: 35905788 Free PMC article. - Endothelial Yes-Associated Protein 1 Promotes Astrocyte Proliferation and Maturation via Cytoplasmic Leukemia Inhibitory Factor Secretion in Oxygen-Induced Retinopathy.
Ai LQ, Zhu JY, Chen X, Li X, Luo LL, Hu QM, Lin S, Ye J. Ai LQ, et al. Invest Ophthalmol Vis Sci. 2020 Apr 9;61(4):1. doi: 10.1167/iovs.61.4.1. Invest Ophthalmol Vis Sci. 2020. PMID: 32271890 Free PMC article. - Nuclear actin regulates cell proliferation and migration via inhibition of SRF and TEAD.
McNeill MC, Wray J, Sala-Newby GB, Hindmarch CCT, Smith SA, Ebrahimighaei R, Newby AC, Bond M. McNeill MC, et al. Biochim Biophys Acta Mol Cell Res. 2020 Jul;1867(7):118691. doi: 10.1016/j.bbamcr.2020.118691. Epub 2020 Feb 28. Biochim Biophys Acta Mol Cell Res. 2020. PMID: 32119877 Free PMC article. - The role of Hippo/yes-associated protein signalling in vascular remodelling associated with cardiovascular disease.
He J, Bao Q, Yan M, Liang J, Zhu Y, Wang C, Ai D. He J, et al. Br J Pharmacol. 2018 Apr;175(8):1354-1361. doi: 10.1111/bph.13806. Epub 2017 May 10. Br J Pharmacol. 2018. PMID: 28369744 Free PMC article. Review.
References
- Southgate K.M., Newby A.C. Serum-induced proliferation of rabbit aortic smooth muscle cells from the contractile state is inhibited by 8-Br-cAMP but not 8-Br-cGMP. Atherosclerosis. 1990;82:113–123. - PubMed
- Wu Y.J., Bond M., Sala-Newby G.B., Newby A.C. Altered S-phase kinase-associated protein-2 levels are a major mediator of cyclic nucleotide-induced inhibition of vascular smooth muscle cell proliferation. Circ. Res. 2006;98:1141–1150. - PubMed
- Palmer D., Maurice D.H. cAMP-mediated inhibition of vascular smooth muscle cell migration: role of cAMP-phosphodiesterases. Mol. Biol. Cell. 1997;8:764.
- Howe A. Regulation of actin-based cell migration by cAMP/PKA. Biochim. Biophys. Acta Mol. Cell Res. 1692;2004:159–174. - PubMed
- Indolfi C., Di Lorenzo E., Rapacciuolo A., Stingone A.M., Stabile E., Leccia A. 8-Chloro-cAMP inhibits smooth muscle cell proliferation in vitro and neointima formation induced by balloon injury in vivo. J. Am. Coll. Cardiol. 2000;36:288–293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- PG/14/82/31126/BHF_/British Heart Foundation/United Kingdom
- PG/15/100/31877/BHF_/British Heart Foundation/United Kingdom
- RG/09/006/27918/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous