Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy - PubMed (original) (raw)
doi: 10.1158/2159-8290.CD-15-0283. Epub 2015 Dec 8.
Jie Qing Chen 1, Chengwen Liu 1, Shruti Malu 1, Caitlin Creasy 1, Michael T Tetzlaff 2, Chunyu Xu 1, Jodi A McKenzie 1, Chunlei Zhang 1, Xiaoxuan Liang 1, Leila J Williams 1, Wanleng Deng 1, Guo Chen 1, Rina Mbofung 1, Alexander J Lazar 3, Carlos A Torres-Cabala 3, Zachary A Cooper 4, Pei-Ling Chen 3, Trang N Tieu 5, Stefani Spranger 6, Xiaoxing Yu 1, Chantale Bernatchez 1, Marie-Andree Forget 1, Cara Haymaker 1, Rodabe Amaria 1, Jennifer L McQuade 7, Isabella C Glitza 1, Tina Cascone 7, Haiyan S Li 8, Lawrence N Kwong 9, Timothy P Heffernan 5, Jianhua Hu 10, Roland L Bassett Jr 10, Marcus W Bosenberg 11, Scott E Woodman 1, Willem W Overwijk 1, Gregory Lizée 1, Jason Roszik 12, Thomas F Gajewski 6, Jennifer A Wargo 4, Jeffrey E Gershenwald 13, Laszlo Radvanyi 1, Michael A Davies 14, Patrick Hwu 14
Affiliations
- PMID: 26645196
- PMCID: PMC4744499
- DOI: 10.1158/2159-8290.CD-15-0283
Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy
Weiyi Peng et al. Cancer Discov. 2016 Feb.
Abstract
T cell-mediated immunotherapies are promising cancer treatments. However, most patients still fail to respond to these therapies. The molecular determinants of immune resistance are poorly understood. We show that loss of PTEN in tumor cells in preclinical models of melanoma inhibits T cell-mediated tumor killing and decreases T-cell trafficking into tumors. In patients, PTEN loss correlates with decreased T-cell infiltration at tumor sites, reduced likelihood of successful T-cell expansion from resected tumors, and inferior outcomes with PD-1 inhibitor therapy. PTEN loss in tumor cells increased the expression of immunosuppressive cytokines, resulting in decreased T-cell infiltration in tumors, and inhibited autophagy, which decreased T cell-mediated cell death. Treatment with a selective PI3Kβ inhibitor improved the efficacy of both anti-PD-1 and anti-CTLA-4 antibodies in murine models. Together, these findings demonstrate that PTEN loss promotes immune resistance and support the rationale to explore combinations of immunotherapies and PI3K-AKT pathway inhibitors.
Significance: This study adds to the growing evidence that oncogenic pathways in tumors can promote resistance to the antitumor immune response. As PTEN loss and PI3K-AKT pathway activation occur in multiple tumor types, the results support the rationale to further evaluate combinatorial strategies targeting the PI3K-AKT pathway to increase the efficacy of immunotherapy.
©2015 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest:
J.A. Wargo has honoraria from speakers’ bureau of Dava Oncology and is an advisory board member for GlaxoSmithKline, Roche/Genentech, and Amgen. M. A. Davies is an advisory board member for GlaxoSmithKline, Roche/Genentech, Novartis, and Sanofi-Aventis, and he has received research funding from GlaxoSmithKline, Roche/Genentech, Sanofi-Aventis, Astrazeneca, Myriad, and Oncothyreon. No potential conflicts of interest were disclosed by other authors.
Figures
Figure 1. Reduced T cell-mediated anti-tumor activity against PTEN-silenced melanoma cells
(A) PTEN expression and AKT activation in A375/GH cells with and without PTEN silencing. Two PTEN-silenced tumor cell lines (17 and 60) were independently produced by two shRNAs targeting PTEN (shPTEN). Tumor cells expression scrambled shRNA (shNS) were served as control (PTEN-WT Tu). (B) T cell-induced apoptosis rate of melanoma tumor cells with and without PTEN silencing. A375/GH/shPTEN and A375/GH/shNS tumor cells were co-cultured with tumor-reactive pmel-1 T cells, at different ratios of effector and target cells (E:T). The cleavage of caspase-3 in tumor cells was determined by flow cytometry. (C) Experimental setup of the murine ACT protocol to evaluate in vivo T cell-mediated anti-tumor activity. (D) T cell infiltration of melanoma tumors with and without PTEN silencing in vivo. Luciferase-expressing pmel-1 T cells were transferred into B6 nude mice bearing A375/GH tumor with or without PTEN-silencing. To evaluate tumor trafficking of transferred T cells, the luciferase intensity at the tumor site was determined by bioluminescence imaging 6 days after T cell transfer. (E) Summary of quantitative imaging analysis of transferred T cells at the tumor site. Quantification was expressed as the average of photon flux within region of interest (ROI). (F) Tumor growth and (G) Kaplan-Meier survival curves for tumor-bearing B6 nude mice treated with adoptive transfer of pmel-1 T cells. Similar results were obtained in repeated experiments. In D–G, three to five mice per group were used. *P < 0.05.
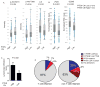
Figure 2. Correlation of PTEN loss in melanoma cells with an immune resistance phenotype
(A) Overview of IHC for PTEN in advanced metastatic melanoma patients. PTEN expression was evaluated by a CLIA-certified PTEN IHC assay. Representative PTEN staining pictures for each category were shown. (B) A waterfall plot of the best objective response in each anti-PD-1 treated patient. 39 cases of melanoma patients treated with anti-PD-1 antibody were stratified based on the expression of PTEN. Tumor burden was measured by the sum of longest diameters of target lesions. The best objective response of anti-PD-1 antibody was evaluated by the maximum change of tumor burden based on baseline. (C) Comparison of tumor reduction after PD-1 therapy in patients with or without PTEN loss. The p value of the comparison was determined by Wilcoxon rank sum test. (D) Increased percentage of PTEN absent in melanoma patients with failed initial expansion of TILs (≤40X106 TIL after initial expansion). A 2X2 contingency table was made based on the frequency distribution of the PTEN expression status and the success of initial TIL growth in the ACT cohort. The p value (p=0.04) was determined by Fisher’s exact t test. (E) Correlation of CD8+ T cell infiltration with PTEN expression status of stage IIIB/C melanoma patient tumors. (F) Examples of CD8 staining in two patients with clonal PTEN expression.
Figure 3. Reduced number and impaired effector functions of TILs in tumors with PTEN deletion or loss-of-function mutations in PTEN
Cutaneous melanoma patients whose information was included in TCGA were stratified based on the PTEN copy number (CN) (cutoff, ≤−0.4, which was chosen in order to maximize the difference in the level of activity of the AKT pathway). A box-and-whisker plot was used to demonstrate the differences in expression levels of indicated genes or proteins between these two groups. (A) Comparison of the intensity of phosphorylated Akt and Lck in melanomas obtained from patients with different PTEN CNs according to RPPA. (B) The mRNA expression levels for genes encoding interferon-γ and granzyme B in tumor samples obtained from melanoma patients with different PTEN CNs. (C) Comparison of lymphocytic infiltration score (Lscore), as determined by pathological review, between groups of patients with different PTEN CNs. The p values of the comparisons were determined by unpaired T test. (D) Frequencies of genetic alterations in the β-catenin pathway and PTEN between T cell-inflamed and non-T cell-inflamed tumors. Metastatic melanomas from TCGA were first catalogued based on T cell infiltration and subsequently based on activating mutations in β-catenin itself (CTNNB1) or loss-of-function mutations in negative regulators of the β-catenin pathway (APC, APC2, AXIN1, AXIN2), and PTEN deletion or PTEN mutations. Non-T cell-inflamed tumors have an increased frequency of PTEN alterations compared to the T cell-inflamed tumors (p<0.01 by Fisher’s exact test).
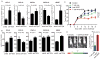
Figure 4. PD-L1 expression is not associated with PTEN expression status in melanomas
(A) The PD-L1 surface expression levels in melanoma cells with and without PTEN silencing. A375 and WM35 tumor cells were transduced with control shRNA or PTEN-specific shRNA. Transduced tumor cells were stained with anti-PD-L1 to evaluate the surface expression of PD-L1. (B) The in vivo PD-L1 expression in melanoma tumor tissues with and without PTEN silencing. B6 nude mice were challenged with A375/GH/shPTEN or A375/GH/shNS tumor cells. Tumor samples were collected from mice bearing 14-day established tumors. The PD-L1 mRNA expression levels in tumor samples were determined using real-time PCR. (C) Examples of IHC for PD-L1 in tumors with clonal PTEN expression. (D) The PD-L1 surface expression levels in tumor samples from patients with stage IIIB/C melanoma. Tumor samples were placed in PTEN absent and PTEN present groups, and the expression of PD-L1 on the tumor surface was determined by IHC.
Figure 5. Critical role of VEGF in immune resistance associated with loss of PTEN
(A and B) B6 nude mice were challenged with A375/GH/shPTEN or A375/GH/shNS melanoma cells and tumor samples were collected from 14-day tumor-bearing mice. (A) Transcript expression levels for cytokine and chemokine genes in tumor samples as determined using quantitative RT-PCR. (B) Protein expression of cytokines and chemokines in tumor samples as determined by Luminex analyses. (C and D) B6 nude mice were challenged with A375/GH/shPTEN. Seven days after tumor challenge, luciferase-expressing pmel-1 T cells were transferred into tumor-bearing mice. Bioluminescence imaging was performed to evaluate tumor trafficking of transferred T cells in treated mice. Tumor growth (C) and T cell infiltration (D) of PTEN-silenced tumor in mice treated with ACT and/or anti-VEGF antibody. Representative imaging figures and quantification of the intensity of luciferase at tumor site from all tested mice were shown. Quantification was expressed as the average of photon flux within ROI. In C–D, three to five mice per group were used. *P < 0.05.
Figure 6. PTEN expression regulates autophagy in tumor cells, reducing T cell-mediated killing
(A) Expression of LC3 I and LC3 II in _BRAF-_mutant melanoma cell lines with and without PTEN silencing. The expression levels of LC3 I and LC3 II in protein lysates from A375 and WM35 tumor cells were determined by western blotting analysis. (B) Perturbing the expression of autophagy related genes changes the sensitivity of melanoma to apoptosis induced by tumor-reactive T cells. A patient-derived melanoma cell line, Mel2400, was transduced with lentiviral vectors encoding the ORFs or shRNAs of autophagy-related genes. Virally transduced tumor cells were co-cultured with paired autologous T cells for 3 hours. The percentage of killed (cleaved casp-3+) tumor cells expressing ORF or shRNAs was determined using flow cytometry. A comboscore was calculated as described in the method section and used to evaluate the effect of genetic modifications on sensitivity of tumor cells to T cell-mediated killing. Tumor cells transduced with virus expressing GFP or shRNA targeting luciferase (Luc) were served as controls for overexpression and knockdown experiments, respectively. *genetic modification, which significantly changed the sensitivity of tumor to T cell-mediated killing (p<0.05). (C) PTEN-silenced and control melanoma cells were transduced with a viral vector encoding MAP1LC3B. The percentage of cleaved casp-3+ cells among transduced tumor cells in response to pmel-1 T cells (E:T=10:1) was evaluated. (D) Melanoma cells (Mel 2338) were pretreated with 1μM Hydroxychloroquine (HCQ) for overnight and followed by co-culture with paired TILs for 3 hours. The percentage of apoptotic tumor cells was evaluated by the cleaved caspase-3 assay. Results are representative of data generated in two independent experiments. *P < 0.05; **P < 0.01; ****P < 0.0001.
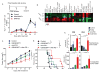
Figure 7. The PI3Kβ inhibitor enhances the anti-tumor activity of T cell-mediated immunotherapy in mice bearing PTEN loss tumor
(A) C57BL/6 mice were transferred with the splenocytes from Pmel-Thy1.1 mice, followed by gp100 peptide vaccination. Vaccinated mice received either vehicle, GSK2636771 (30mg/kg/d), or BKM120 (60mg/kg/d) for 5 days. After 30 days, mice were boosted with gp100 peptide vaccine. Schematic representation of vaccine and the PI3K inhibitor (PI3Ki) treatment protocol was shown. Thy1.1, a congenic marker for transferred Pmel T cells, was used to determine the number of gp100-specific T cells in peripheral blood from vaccinated mice after the PI3K inhibitor treatment. (B) Analysis of the effect of GSK2636771 on protein signaling networks by RPPA. Melanoma was initiated in a group of Tyr:CreER; PTENlox/lox; BRAF V600E/+ mice. Mice with measureable tumors were randomly treated with either vehicle or GSK2636771 (30mg/kg/d) for 5 days. Protein lysates from treated tumors were harvested. The heatmap demonstrates the changes in proteins differentially expressed in GSK2636771 treated tumors. (C–D) Tyr:CreER; PTENlox/lox; BRAF V600E/+ mice with measureable tumors were randomly treated with either vehicle plus control antibody, GSK2636771 (30mg/kg/d), anti-PD-1(100μg), or the combination of both GSK2636771 and anti-PD-1. (C) Tumor size in each of the treatment groups. Tumor growth was monitored every 3 days. (D) Kaplan-Meier survival curves of mice treated with GSK2636771 and/or anti-PD-1. Log-rank test demonstrates statistical significance (P<0.05): control vs GSK2636771 or GSK2636771+anti-PD-1; GSK2636771 vs GSK2636771+anti-PD-1; anti-PD-1 vs GSK2636771+anti-PD-1 (N=4–8). (E) The numbers of tumor-infiltrating T cells in mice treated with GSK2636771 and/or anti-PD-1. GSK2636771 and/or anti-PD-1 were used to treat mice bearing either a spontaneous tumor or a transplanted tumor as described in Figure 7C and Supplementary Figure S7D respectively. 7 days after treatment, tumor tissues were harvested and weighed. Single cell suspensions from tumor tissues were made for CD8 and CD4 staining. One-Way ANOVA test demonstrates statistical significance (P<0.05): GSK2636771+anti-PD-1 vs control, GSK2636771 or anti-PD-1. *P < 0.05(N=4–5).
Similar articles
- Secondary resistance to immunotherapy associated with β-catenin pathway activation or PTEN loss in metastatic melanoma.
Trujillo JA, Luke JJ, Zha Y, Segal JP, Ritterhouse LL, Spranger S, Matijevich K, Gajewski TF. Trujillo JA, et al. J Immunother Cancer. 2019 Nov 8;7(1):295. doi: 10.1186/s40425-019-0780-0. J Immunother Cancer. 2019. PMID: 31703593 Free PMC article. - Anti-OX40 Antibody Directly Enhances The Function of Tumor-Reactive CD8+ T Cells and Synergizes with PI3Kβ Inhibition in PTEN Loss Melanoma.
Peng W, Williams LJ, Xu C, Melendez B, McKenzie JA, Chen Y, Jackson HL, Voo KS, Mbofung RM, Leahey SE, Wang J, Lizee G, Tawbi HA, Davies MA, Hoos A, Smothers J, Srinivasan R, Paul EM, Yanamandra N, Hwu P. Peng W, et al. Clin Cancer Res. 2019 Nov 1;25(21):6406-6416. doi: 10.1158/1078-0432.CCR-19-1259. Epub 2019 Aug 1. Clin Cancer Res. 2019. PMID: 31371342 Free PMC article. - PTEN loss correlates with T cell exclusion across human cancers.
Lin Z, Huang L, Li SL, Gu J, Cui X, Zhou Y. Lin Z, et al. BMC Cancer. 2021 Apr 19;21(1):429. doi: 10.1186/s12885-021-08114-x. BMC Cancer. 2021. PMID: 33874915 Free PMC article. - Coinhibitory Pathways in Immunotherapy for Cancer.
Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Baumeister SH, et al. Annu Rev Immunol. 2016 May 20;34:539-73. doi: 10.1146/annurev-immunol-032414-112049. Epub 2016 Feb 25. Annu Rev Immunol. 2016. PMID: 26927206 Review. - A Systematic Review of Immunotherapy in Urologic Cancer: Evolving Roles for Targeting of CTLA-4, PD-1/PD-L1, and HLA-G.
Carosella ED, Ploussard G, LeMaoult J, Desgrandchamps F. Carosella ED, et al. Eur Urol. 2015 Aug;68(2):267-79. doi: 10.1016/j.eururo.2015.02.032. Epub 2015 Mar 29. Eur Urol. 2015. PMID: 25824720 Review.
Cited by
- Notch1 blockade by a novel, selective anti-Notch1 neutralizing antibody improves immunotherapy efficacy in melanoma by promoting an inflamed TME.
de Freitas JT, Thakur V, LaPorte KM, Thakur VS, Flores B, Caicedo V, Ajaegbu CGE, Ingrasci G, Lipman ZM, Zhang K, Qiu H, Malek TR, Bedogni B. de Freitas JT, et al. J Exp Clin Cancer Res. 2024 Nov 4;43(1):295. doi: 10.1186/s13046-024-03214-5. J Exp Clin Cancer Res. 2024. PMID: 39491031 Free PMC article. - Cold and hot tumors: from molecular mechanisms to targeted therapy.
Wu B, Zhang B, Li B, Wu H, Jiang M. Wu B, et al. Signal Transduct Target Ther. 2024 Oct 18;9(1):274. doi: 10.1038/s41392-024-01979-x. Signal Transduct Target Ther. 2024. PMID: 39420203 Free PMC article. Review. - Targeting IRE1α reprograms the tumor microenvironment and enhances anti-tumor immunity in prostate cancer.
Unal B, Kuzu OF, Jin Y, Osorio D, Kildal W, Pradhan M, Kung SHY, Oo HZ, Daugaard M, Vendelbo M, Patterson JB, Thomsen MK, Kuijjer ML, Saatcioglu F. Unal B, et al. Nat Commun. 2024 Oct 15;15(1):8895. doi: 10.1038/s41467-024-53039-1. Nat Commun. 2024. PMID: 39406723 Free PMC article. - Alpelisib and Immunotherapy: A Promising Combination for Recurrent and Metastatic Squamous Cell Carcinoma of the Head and Neck.
Suleiman R, McGarrah P, Baral B, Owen D, Vera Aguilera J, Halfdanarson TR, Price KA, Fuentes Bayne HE. Suleiman R, et al. Cancer Rep (Hoboken). 2024 Oct;7(10):e70023. doi: 10.1002/cnr2.70023. Cancer Rep (Hoboken). 2024. PMID: 39376013 Free PMC article. - Current progress of anti‑PD‑1/PDL1 immunotherapy for glioblastoma (Review).
Wu J, Wang N. Wu J, et al. Mol Med Rep. 2024 Dec;30(6):221. doi: 10.3892/mmr.2024.13344. Epub 2024 Oct 4. Mol Med Rep. 2024. PMID: 39364736 Free PMC article. Review.
References
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA128913/CA/NCI NIH HHS/United States
- P01CA128913/CA/NCI NIH HHS/United States
- U54 CA163125/CA/NCI NIH HHS/United States
- R01 CA154710/CA/NCI NIH HHS/United States
- R01 CA116206/CA/NCI NIH HHS/United States
- P30CA016672/CA/NCI NIH HHS/United States
- R00 CA204595/CA/NCI NIH HHS/United States
- P50CA093459/CA/NCI NIH HHS/United States
- P30 CA016359/CA/NCI NIH HHS/United States
- 1K08CA160692-01A1/CA/NCI NIH HHS/United States
- R01 CA187076/CA/NCI NIH HHS/United States
- P50 CA093459/CA/NCI NIH HHS/United States
- R01CA154710/CA/NCI NIH HHS/United States
- R01CA116206/CA/NCI NIH HHS/United States
- K08 CA160692/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- K99 CA204595/CA/NCI NIH HHS/United States
- T32 CA009666/CA/NCI NIH HHS/United States
- UL1 TR000371/TR/NCATS NIH HHS/United States
- U54CA163125-01/CA/NCI NIH HHS/United States
- R01CA187076/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials