Up-Regulatory Effects of Curcumin on Large Conductance Ca2+-Activated K+ Channels - PubMed (original) (raw)
Up-Regulatory Effects of Curcumin on Large Conductance Ca2+-Activated K+ Channels
Qijing Chen et al. PLoS One. 2015.
Abstract
Large conductance Ca2+-activated potassium channels (BK) are targets for research that explores therapeutic means to various diseases, owing to the roles of the channels in mediating multiple physiological processes in various cells and tissues. We investigated the pharmacological effects of curcumin, a compound isolated from the herb Curcuma longa, on BK channels. As recorded by whole-cell patch-clamp, curcumin increased BK (α) and BK (α+β1) currents in transfected HEK293 cells as well as the current density of BK in A7r5 smooth muscle cells in a dose-dependent manner. By incubating with curcumin for 24 hours, the current density of exogenous BK (α) in HEK293 cells and the endogenous BK in A7r5 cells were both enhanced notably, though the steady-state activation of the channels did not shift significantly, except for BK (α+β1). Curcumin up-regulated the BK protein expression without changing its mRNA level in A7r5 cells. The surface expression and the half-life of BK channels were also increased by curcumin in HEK293 cells. These effects of curcumin were abolished by MG-132, a proteasome inhibitor. Curcumin also increased ERK 1/2 phosphorylation, while inhibiting ERK by U0126 attenuated the curcumin-induced up-regulation of BK protein expression. We also observed that the curcumin-induced relaxation in the isolated rat aortic rings was significantly attenuated by paxilline, a BK channel specific blocker. These results show that curcumin enhances the activity of the BK channels by interacting with BK directly as well as enhancing BK protein expression through inhibiting proteasomal degradation and activating ERK signaling pathway. The findings suggest that curcumin is a potential BK channel activator and provide novel insight into its complicated pharmacological effects and the underlying mechanisms.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
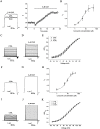
Fig 1. Effect of curcumin on exogenous BK (α) channel currents in HEK293 cells.
(A) Representative whole cell current traces from HEK293 cells expressing BK channels before and after the perfusion of 5 μM curcumin (left) and the time course of the enhancement on BK (α) channels by of curcumin (right). With 3 μM free Ca2+ in the pipette solution, the holding voltage was controlled at -80 mV and BK currents were evoked by the pulse of +100 mV. (B) The dose-dependence curve of curcumin enhancing BK currents was fitted by the Hill equation (see “Data analysis”). The EC50 value is 5.83±0.76 μM with a Hill coefficient of n = 1.71±0.30 (n = 8). (C) HEK293 cells were held at -80 mV, and the duration of 200 ms voltage steps were applied from -50 to +120 mV at 10 mV increments. Representative trace before treatment with curcumin was shown. (D) Representative trace manifests the effect of curcumin at 5 μM. (E) The plots of the normalized conductance were fitted well with Boltzmann function (“see Data analysis”). The voltage dependence of steady state activation curve was not shifted significantly in the presence of curcumin (n = 8). □ represents the curve of BK channels before exposure to curcumin. ○ represents the curve of BK channels after exposure to 5 μM curcumin. Δ represents the curve of BK channels after exposure to 20 μM curcumin. Representative whole cell current traces from HEK293 cells expressing BK channels before (F) and after (G) the perfusion of curcumin 5 μM for 24 hours. (H) The dose-dependence curve of enhanced BK current density by curcumin was fitted by the Hill equation (see “Data analysis”). The EC50 value is 8.05±0.97 μM with a Hill co-efficient of n = 1.77±0.45 (n = 5). (I) Representative trace of BK currents and (J) the trace manifested the effect of curcumin at the concentration of 5 μM. (K) The plots of the normalized conductance were fitted well with Boltzmann function (“see Data analysis”). The voltage dependence of steady state activation curve was not shifted significantly in the presence of curcumin (n = 9). □ represents the curve of BK channels before exposure to curcumin. ○ represents the curve of BK channels after exposure to 5 μM curcumin. Δ represents the curve of BK channels after exposure to 20 μM curcumin.
Fig 2. Effect of curcumin on exogenous BK (α+β1) channel currents in HEK293 cells.
(A) Representative whole cell current traces from HEK293 cells expressing BK (α+β1) channels before and after the perfusion of 5 μM curcumin are presented at left and the time course for the enhancement on BK (α+β1) channels by curcumin at right. With 3 μM free Ca2+ in the pipette solution, the holding voltage was controlled at -80 mV and the BK currents were evoked by the pulse of +100 mV. (B) The dose-dependence curve of curcumin enhanced BK (α+β1) currents was fitted by the Hill equation (see “Data analysis”). The EC50 value is 4.02±0.67 μM with a Hill coefficient of n = 2.31±0.58 (n = 5). (C) HEK293 cells were held at -80 mV, and the duration of 200 ms voltage steps were applied from -50 to +120 mV at 10 mV increments. Representative trace before treatment with curcumin was shown. (D) Representative trace manifestes the effect of curcumin at the concentration of 5 μM. (E) The plots of the normalized conductance were fitted well with Boltzmann function (“see Data analysis”). The voltage dependence of steady state activation curve was not shifted significantly in the presence of curcumin (n = 5). □ represents the curve of BK (α+β1) channels before exposure to curcumin. ○ represents the curve of BK (α+β1) channels after exposure to 5 μM curcumin. Δ represents the curve of BK (α+β1) channels after exposure to 20 μM curcumin.
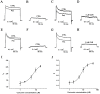
Fig 3. Effect of curcumin on the current density of endogenous BK channels in A7r5 cells.
(A) Representative traces of whole cell currents from A7r5 cells in the absence of curcumin before and after application of 10 μM paxilline. The holding potential was -80 mV and the currents were evoked by +120 mV with 3 μM free Ca2+ in the pipette solution. (B) Representative traces of paxilline-sensitive currents in A7r5 cells. (C) The whole cell current traces of A7r5 cells in the presence of curcumin before and after application of 10 μM paxilline. (D) Representative traces of paxilline-sensitive currents in the presence of curcumin. (E) Representative traces of whole cell currents from A7r5 cells in the absence of curcumin before and after application of 10 μM paxilline. (F) Representative traces of paxilline-sensitive currents in A7r5 cells. (G) The whole cell current traces of A7r5 cells in the presence of curcumin for 24 hr before and after application of 10 μM paxilline. (H) Representative traces of paxilline-sensitive currents in the presence of curcumin for 24 hr. (I) The dose-dependence curve of curcumin enhancing current density of BK channels was fitted by the Hill equation (see “Data analysis”). The EC50 value was assessed to be 6.93±0.78 μM with a Hill coefficient of n = 2.00±0.55 (n = 6). (J) The dose-dependence curve of curcumin enhancing current density of BK channels was fitted by the Hill equation (see “Data analysis”). The EC50 value is 7.36±0.10 with Hill coefficient 2.19±0.05 (n = 6).
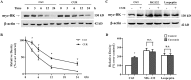
Fig 4. Curcumin increased the surface and total BK protein expressions in HEK293 cells.
(A) Dose-dependent and time-dependent effects of curcumin on the BK protein in transfected HEK293 cells. Western blot analysis indicated that treatment with 2.5 to 20 μM curcumin for 24 h increased BK protein level in a concentration-dependent manner (n = 5). (B) The BK protein expression in HEK293 cells incubated with 10 μM curcumin for the time course of 0.5h to 48 h, showing an increase of BK protein level in response to curcumin (n = 3). Graphic representative of densitometric data of the total BK protein level shown in (C) and (D). (E) Representative western blot of total and surface BK protein expressions by cell surface biotinylation. The densitometric data showing the surface (F) and total (G) BK protein level and ratio of surface over total BK protein level (H) (n = 3). * p <0.05; n.s., not significant.
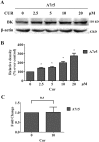
Fig 5. Effect of curcumin on BK protein abundance and BK mRNA in A7r5 cells.
(A) Western blot analysis showed that treatment with curcumin at 0, 2.5, 5, 10, and 20 μM for 24h up-regulated the BK protein expression in A7r5 cells. * p <0.05 (n = 3). Graphic representative of densitometric data of the above total BK protein level are shown in (B). (C) Real-time RT-PCR for the measurement of BK mRNA levels with 10 μM curcumin treatment; the BK mRNA levels were normalized with GAPDH. The predicted amplicon size for BK and GAPDH is 103 bp and 271 bp, respectively. n.s., not significant (n = 3).
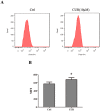
Fig 6. Effect of curcumin on intracellular free Ca2+ concentrations in HEK 293 cells.
After treatment with 0 or 10 μM curcumin for 24 h, intracellular free Ca2+ were determined by flow-cytometric analysis stained with Fluo-3AM for 30 min. Results are expressed as mean fluorescent intensity.
Fig 7. Curcumin increased BK protein stability via inhibition of degradation pathways.
(A) Effect of curcumin on BK protein stability was examined with cycloheximide-chase assay. Western blot analysis showed that treatment with 10μM curcumin prolonged BK protein half-life. (B) Graphic representation of densitometric data showing the remaining BK protein level in Fig 7A (n = 3). (C) Effect of curcumin on BK protein in the presence and absence of proteasomal inhibitor, MG132. Western blot analysis showed that the increase of BK protein level was abolished by the proteasome inhibitor, MG132 (5 μM). Inhibitors were applied 1 h before the administration of curcumin. (D) Graphic representation of densitometric data of the BK protein level in Fig 7C (n = 3). * p < 0.05; n.s., not significant.
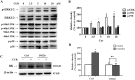
Fig 8. Curcumin up-regulated BK protein expression via ERK1/2 signaling pathway.
(A) Effects of curcumin on ERK1/2, JNK, p38 phosphorylation. HEK293 cells were incubated with 10μM curcumin for 24 h, and the phosphorylation of ERK1/2, JNK, p38 were analyzed by Western Blot. (B) Graphic representation of densitometric data of the phosphorylation level of ERK1/2 (n = 3). (C) Western Blot analysis showed that the ERK1/2 inhibitors, U0126 (10 μM), abolished the effect of curcumin on BK protein. Inhibitors were applied 1 h before administration of curcumin. (D) Graphic representation of densitometric data show a protein level of BK (n = 4). * p < 0.05; ** p < 0.01; n.s., not significant.
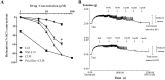
Fig 9. Effect of curcumin on the relaxation of the isolated rat aortic rings.
(A) Concentration—response curves for the control, NS1619, curcumin (CUR) and paxalline (BK blocker) + CUR groups in the 20mM KCl-contracted arotic rings. Vasorelaxant effects are expressed as the percentage of the steady-state tension (100%) obtained with 20 mM KCl. Data points are presented as mean ± SE. (n = 4). *p < 0.05 compared to curcumin treatment alone. (B) Representative original traces of the vasorelaxant effects of curcumin, recorded on aortic rings precontracted with 20 mM KCl, in the absence or in the presence of paxilline. The curcumin was added cumulatively at the concentrations of 1, 5, 10, 20, 40 μM.
Similar articles
- G protein pathway suppressor 2 enhanced the renal large-conductance Ca2+-activated potassium channel expression via inhibiting ERK1/2 signaling pathway.
Zhuang Z, Xiao J, Chen X, Hu X, Li R, Chen S, Feng X, Shen S, Ma HP, Zhuang J, Cai H. Zhuang Z, et al. Am J Physiol Renal Physiol. 2018 Sep 1;315(3):F503-F511. doi: 10.1152/ajprenal.00041.2018. Epub 2018 May 16. Am J Physiol Renal Physiol. 2018. PMID: 29767559 - Tyrphostin AG556 increases the activity of large conductance Ca2+ -activated K+ channels by inhibiting epidermal growth factor receptor tyrosine kinase.
Wang Y, Sun HY, Liu YG, Song Z, She G, Xiao GS, Wang Y, Li GR, Deng XL. Wang Y, et al. J Cell Mol Med. 2017 Sep;21(9):1826-1834. doi: 10.1111/jcmm.13103. Epub 2017 Mar 14. J Cell Mol Med. 2017. PMID: 28294531 Free PMC article. - Daidzein relaxes rat cerebral basilar artery via activation of large-conductance Ca2+-activated K+ channels in vascular smooth muscle cells.
Zhang HT, Wang Y, Deng XL, Dong MQ, Zhao LM, Wang YW. Zhang HT, et al. Eur J Pharmacol. 2010 Mar 25;630(1-3):100-6. doi: 10.1016/j.ejphar.2009.12.032. Epub 2010 Jan 5. Eur J Pharmacol. 2010. PMID: 20044987 - Regulation of large conductance Ca2+-activated K+ (BK) channel β1 subunit expression by muscle RING finger protein 1 in diabetic vessels.
Yi F, Wang H, Chai Q, Wang X, Shen WK, Willis MS, Lee HC, Lu T. Yi F, et al. J Biol Chem. 2014 Apr 11;289(15):10853-10864. doi: 10.1074/jbc.M113.520940. Epub 2014 Feb 25. J Biol Chem. 2014. PMID: 24570002 Free PMC article. - Effects of curcumin on ion channels and transporters.
Zhang X, Chen Q, Wang Y, Peng W, Cai H. Zhang X, et al. Front Physiol. 2014 Mar 11;5:94. doi: 10.3389/fphys.2014.00094. eCollection 2014. Front Physiol. 2014. PMID: 24653706 Free PMC article. Review.
Cited by
- BK Knockout by TALEN-Mediated Gene Targeting in Osteoblasts: KCNMA1 Determines the Proliferation and Differentiation of Osteoblasts.
Hei H, Gao J, Dong J, Tao J, Tian L, Pan W, Wang H, Zhang X. Hei H, et al. Mol Cells. 2016 Jul;39(7):530-5. doi: 10.14348/molcells.2016.0033. Epub 2016 Jun 21. Mol Cells. 2016. PMID: 27329042 Free PMC article. - Activating BK channels ameliorates vascular smooth muscle calcification through Akt signaling.
Ning FL, Tao J, Li DD, Tian LL, Wang ML, Reilly S, Liu C, Cai H, Xin H, Zhang XM. Ning FL, et al. Acta Pharmacol Sin. 2022 Mar;43(3):624-633. doi: 10.1038/s41401-021-00704-6. Epub 2021 Jun 23. Acta Pharmacol Sin. 2022. PMID: 34163023 Free PMC article. - Development of charybdotoxin Q18F variant as a selective peptide blocker of neuronal BK(α + β4) channel for the treatment of epileptic seizures.
Liu X, Tao J, Zhang S, Lan W, Yao Y, Wang C, Xue H, Ji Y, Li G, Cao C. Liu X, et al. Protein Sci. 2022 Dec;31(12):e4506. doi: 10.1002/pro.4506. Protein Sci. 2022. PMID: 36369672 Free PMC article. - Evolution of Natural Product Scaffolds as Potential Proteasome Inhibitors in Developing Cancer Therapeutics.
Mir RH, Mir PA, Uppal J, Chawla A, Patel M, Bardakci F, Adnan M, Mohi-Ud-Din R. Mir RH, et al. Metabolites. 2023 Mar 31;13(4):509. doi: 10.3390/metabo13040509. Metabolites. 2023. PMID: 37110167 Free PMC article. Review. - Modulatory effects of bufalin, an active ingredient from toad venom on voltage-gated sodium channels.
Tao J, Jiang F, Liu C, Liu Z, Zhu Y, Xu J, Ge Y, Xu K, Yin P. Tao J, et al. Mol Biol Rep. 2018 Oct;45(5):721-740. doi: 10.1007/s11033-018-4213-9. Epub 2018 Jun 21. Mol Biol Rep. 2018. PMID: 29931533
References
- Kunzelmann K. Ion channels and cancer. J Membrane Biol. 2005;205(3):159–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous