Characterization of mannanase from Bacillus circulans NT 6.7 and its application in mannooligosaccharides preparation as prebiotic - PubMed (original) (raw)
Characterization of mannanase from Bacillus circulans NT 6.7 and its application in mannooligosaccharides preparation as prebiotic
Phanwipa Pangsri et al. Springerplus. 2015.
Abstract
This study focused on the characterization of mannanase from Bacillus circulans NT 6.7 for mannooligosaccharides (MOS) production. The enzyme from B. circulans NT 6.7 was produced using defatted copra meal as a carbon source. The mannanase was purified by ultrafiltration and column chromatography of Q-Sepharose. The purified protein (M1) was a dimeric protein with a 40 kDa subunit. The purified M1 exhibited optimum pH and temperature at pH 6.0 and 60 °C, respectively. It was activated by Mn(2+,) Mg(2+,) and Cu(2+), and as inhibited by EDTA (45-65 %). The purified enzyme exhibited high specificity to beta-mannan: konjac (glucomannan), locust bean gum (galactomannan), ivory nut (mannan), guar gum (galactomannan) and defatted copra meal (galactomannan). The defatted copra meal could be hydrolyzed by purified M1 into mannooligosaccharides which promoted beneficial bacteria, especially Lactobacillus group, and inhibited pathogenic bacteria; Shigella dysenteria DMST 1511, Staphylococcus aureus TISTR 029, and Salmonella enterica serovar Enteritidis DMST 17368. Therefore, the mannanase from B. circulans NT 6.7 would be a novel source of enzymes for the mannooligosaccharides production as prebiotics.
Keywords: Bacillus circulans; Defatted copra meal; Mannanase; Mannooligosaccharides; Prebiotics.
Figures
Fig. 1
Zymogram analysis and SDS-PAGE of purified M1 a zymogram analysis: lane 1 crude enzyme lane 2, purified M1; b SDS-PAGE: lane M Marker (Bio-Rad, USA) lane 1 crude enzyme lane 2 purified M1
Fig. 2
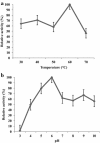
Effect of temperature (a) and pH (b) on purified M1
Fig. 3
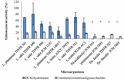
Enhancement properties of DCM-hydrolysate prepared with the purified M1 on beneficial bacteria
Fig. 4
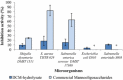
Inhibition properties of DCM-hydrolysate prepared with the purified M1 on pathogenic bacteria
Similar articles
- Cloning, secretory expression and characterization of recombinant β-mannanase from Bacillus circulans NT 6.7.
Piwpankaew Y, Sakulsirirat S, Nitisinprasert S, Nguyen TH, Haltrich D, Keawsompong S. Piwpankaew Y, et al. Springerplus. 2014 Aug 13;3:430. doi: 10.1186/2193-1801-3-430. eCollection 2014. Springerplus. 2014. PMID: 25157333 Free PMC article. - Optimization of hydrolysis conditions for the mannooligosaccharides copra meal hydrolysate production.
Rungruangsaphakun J, Keawsompong S. Rungruangsaphakun J, et al. 3 Biotech. 2018 Mar;8(3):169. doi: 10.1007/s13205-018-1178-2. Epub 2018 Mar 9. 3 Biotech. 2018. PMID: 29527456 Free PMC article. - Optimized production and characterization of endo-β-mannanase by Aspergillus niger for generation of prebiotic mannooligosaccharides from guar gum.
Nath S, Kango N. Nath S, et al. Sci Rep. 2024 Jun 18;14(1):14015. doi: 10.1038/s41598-024-63803-4. Sci Rep. 2024. PMID: 38890382 Free PMC article. - Functional β-mannooligosaccharides: Sources, enzymatic production and application as prebiotics.
Rana M, Jassal S, Yadav R, Sharma A, Puri N, Mazumder K, Gupta N. Rana M, et al. Crit Rev Food Sci Nutr. 2024;64(28):10221-10238. doi: 10.1080/10408398.2023.2222165. Epub 2023 Jun 19. Crit Rev Food Sci Nutr. 2024. PMID: 37335120 Review. - Enzymatic Conversion of Mannan-Rich Plant Waste Biomass into Prebiotic Mannooligosaccharides.
Hlalukana N, Magengelele M, Malgas S, Pletschke BI. Hlalukana N, et al. Foods. 2021 Aug 26;10(9):2010. doi: 10.3390/foods10092010. Foods. 2021. PMID: 34574120 Free PMC article. Review.
Cited by
- Nutritional improvement of copra meal using mannanase and Saccharomyces cerevisiae.
Kraikaew J, Morakul S, Keawsompong S. Kraikaew J, et al. 3 Biotech. 2020 Jun;10(6):274. doi: 10.1007/s13205-020-02271-9. Epub 2020 May 29. 3 Biotech. 2020. PMID: 32523868 Free PMC article. - In vitro fermentation of copra meal hydrolysate by chicken microbiota.
Prayoonthien P, Nitisinprasert S, Keawsompong S. Prayoonthien P, et al. 3 Biotech. 2018 Jan;8(1):41. doi: 10.1007/s13205-017-1058-1. Epub 2017 Dec 29. 3 Biotech. 2018. PMID: 29291154 Free PMC article. - In vitro fermentation of copra meal hydrolysate by human fecal microbiota.
Prayoonthien P, Rastall RA, Kolida S, Nitisinprasert S, Keawsompong S. Prayoonthien P, et al. 3 Biotech. 2019 Mar;9(3):93. doi: 10.1007/s13205-019-1633-8. Epub 2019 Feb 19. 3 Biotech. 2019. PMID: 30800604 Free PMC article. - Copra meal hydrolysis by the recombinant β-mannanase KMAN-3 and MAN 6.7 expressed in Escherichia coli.
Sritrakul N, Nitisinprasert S, Keawsompong S. Sritrakul N, et al. 3 Biotech. 2020 Feb;10(2):44. doi: 10.1007/s13205-019-2005-0. Epub 2020 Jan 11. 3 Biotech. 2020. PMID: 31988838 Free PMC article. - Cloning, secretory expression and characterization of recombinant β-mannanase from Bacillus circulans NT 6.7.
Piwpankaew Y, Sakulsirirat S, Nitisinprasert S, Nguyen TH, Haltrich D, Keawsompong S. Piwpankaew Y, et al. Springerplus. 2014 Aug 13;3:430. doi: 10.1186/2193-1801-3-430. eCollection 2014. Springerplus. 2014. PMID: 25157333 Free PMC article.
References
- Agnes W, Domig KJ, Kneifel W. Comparison of selective media for the enumeration of probiotic enterococci from animal feed. Food Technol Biotechnol. 2005;43(2):147–155.
- Ferket PR, Parks CW, Grimes JL (2002) Benefits of dietary antibiotic and mannan oligosaccharide supplementation for poultry. In: Proceedings multi-state poultry feeding and nutrition conference, Indianapolis Indiana USA, 14–16 May
- Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1402. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials