Critical role of RAGE and HMGB1 in inflammatory heart disease - PubMed (original) (raw)
. 2016 Jan 12;113(2):E155-64.
doi: 10.1073/pnas.1522288113. Epub 2015 Dec 29.
Martin Andrassy 1, Anna-Maria Müller 1, Mariella Bockstahler 1, Andrea Fischer 1, Christian H Volz 1, Christoph Leib 1, Stefan Göser 1, Sevil Korkmaz-Icöz 2, Stefan Zittrich 3, Andreas Jungmann 1, Felix Lasitschka 4, Gabriele Pfitzer 3, Oliver J Müller 5, Hugo A Katus 5, Ziya Kaya 6
Affiliations
- PMID: 26715748
- PMCID: PMC4720305
- DOI: 10.1073/pnas.1522288113
Critical role of RAGE and HMGB1 in inflammatory heart disease
Anna Bangert et al. Proc Natl Acad Sci U S A. 2016.
Abstract
Autoimmune response to cardiac troponin I (TnI) induces inflammation and fibrosis in the myocardium. High-mobility group box 1 (HMGB1) is a multifunctional protein that exerts proinflammatory activity by mainly binding to receptor for advanced glycation end products (RAGE). The involvement of the HMGB1-RAGE axis in the pathogenesis of inflammatory cardiomyopathy is yet not fully understood. Using the well-established model of TnI-induced experimental autoimmune myocarditis (EAM), we demonstrated that both local and systemic HMGB1 protein expression was elevated in wild-type (wt) mice after TnI immunization. Additionally, pharmacological inhibition of HMGB1 using glycyrrhizin or anti-HMGB1 antibody reduced inflammation in hearts of TnI-immunized wt mice. Furthermore, RAGE knockout (RAGE-ko) mice immunized with TnI showed no structural or physiological signs of cardiac impairment. Moreover, cardiac overexpression of HMGB1 using adeno-associated virus (AAV) vectors induced inflammation in the hearts of both wt and RAGE-ko mice. Finally, patients with myocarditis displayed increased local and systemic HMGB1 and soluble RAGE (sRAGE) expression. Together, our study highlights that HMGB1 and its main receptor, RAGE, appear to be crucial factors in the pathogenesis of TnI-induced EAM, because inhibition of HMGB1 and ablation of RAGE suppressed inflammation in the heart. Moreover, the proinflammatory effect of HMGB1 is not necessarily dependent on RAGE only. Other receptors of HMGB1 such as Toll-like receptors (TLRs) may also be involved in disease pathogenesis. These findings could be confirmed by the clinical relevance of HMGB1 and sRAGE. Therefore, blockage of one of these molecules might represent a novel therapeutic strategy in the treatment of autoimmune myocarditis and inflammatory cardiomyopathy.
Keywords: AAV; cytokines; myocarditis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
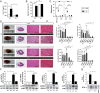
TnI immunization increased HMGB1 protein level and activated specific signal transduction pathways in myocardium of wt mice. (A) Myocardial histoscore of CFA- or TnI-immunized mice for inflammation (HE), fibrosis (MT), and HMGB1 expression on days 21, 90, and 270. (B) Corresponding macroscopic pictures (Left) and histopathological examinations (Right) of immunized mice. [Scale bars, 100 µm (columns 2–4) and 25 µm (column 5).] (C) Immunoblot and fold increase of HMGB1 compared with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in cardiac proteins of TnI-immunized mice on days 0, 21, 90, and 270. (D) Serum HMGB1 level in immunized mice. (E and F) Immunoblot and fold increase of p-STAT3 compared with t-STAT3 (E) and p-JNK compared with t-JNK (F) in cardiac proteins of TnI-immunized mice on days 0, 21, 90, and 270. Error bars indicate mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.005.
Fig. S1.
Experimental setup. Further details are displayed in Table S1.
Fig. 2.
HMGB1 inhibition decreased TnI-induced myocardial inflammation. Mice were immunized with CFA or TnI and treated daily (GL21) or from day 14 to 21 (GL14) with glycyrrhizin. (A) hs-TnT levels in serum of immunized mice treated with glycyrrhizin or control buffer (PBS). (B) Ejection fraction. (C) Myocardial inflammation score was determined using HE staining on day 21. (D) hs-TnT levels in serum of immunized mice treated with anti-HMGB1 antibody or control antibody. (E) Ejection fraction. (F) Histoscore of inflammation was determined using HE staining on day 21. Error bars indicate mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.005.
Fig. 3.
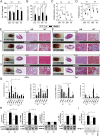
RAGE-ko mice were protected from developing TnI-induced EAM. Wt and RAGE-ko mice were immunized with CFA or TnI. Measurements were done on day 21. (A) hs-TnT levels in serum of immunized mice. (B) Ejection fraction. (C) Histoscore of inflammation and fibrosis in the hearts of immunized mice. (D) Representative macroscopic pictures (Left) and histopathological examinations (Right) of hearts stained with HE and MT. [Scale bars, 3 mm (column 2) and 100 µm (columns 3 and 4).] (E) Myocardial mRNA levels of genes involved in cardiac inflammation. (F) Heart tissues were analyzed by Western blot, and fold increase of phosphorylated compared with total STAT3, JAK2, ERK1/2, and JNK proteins was thereby detected. (G) Electrophoretic mobility shift assay was performed to measure the NF-κB binding activity in myocardium. Specificity of NF-κB binding activity was shown by including a 160-fold molar excess of unlabeled consensus NF-κB oligonucleotide (Cons.). Fold increase of NF-κB compared to Cons. was calculated. Error bars indicate mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.005.
Fig. S2.
HMGB1 levels in the myocardium and serum of wt and RAGE-ko mice after AAV9-vector treatment. Wt and RAGE-ko mice were treated with AAV9 vectors (HMGB1 or Luc) and immunized only with CFA. Measurements were done on day 21. (A) Myocardial HMGB1 protein levels were increased in HMGB1-AAV–treated wt and RAGE-ko compared with Luc-AAV mice. (B) HMGB1 levels in serum were increased in HMGB1-AAV–treated wt and RAGE-ko compared with Luc-AAV mice. Error bars indicate mean ± SEM. *P < 0.05.
Fig. 4.
HMGB1 treatment induced cardiac damage and affected activation of inflammation-related genes and signaling cascades in wt and RAGE-ko mice. Wt and RAGE-ko mice were treated with AAV9-HMGB1 or control vector (Luc) and were immunized with CFA or TnI. Measurements were done on day 21. (A) hs-TnT production in serum of immunized mice upon AAV9-vector treatment. (B) Ejection fraction. (C) Inflammation. (D) Fibrosis score. (E and F) Representative macroscopic pictures (Left) and histopathological examinations (Right) of hearts stained with HE and MT. [Scale bars, 3 mm (column 2) and 100 µm (columns 3 and 4).] (G) Myocardial mRNA levels were analyzed by qPCR. (H) Heart tissues were analyzed by Western blot, and fold increase of phosphorylated compared with total STAT3, JAK2, ERK1/2, and JNK proteins was thereby detected. (I) Electrophoretic mobility shift assay was performed to measure the NF-κB binding activity in myocardium. Specificity of NF-κB binding activity was shown by including a 160-fold molar excess of unlabeled consensus NF-κB oligonucleotide (Cons.). Fold increase of NFkB compared to Cons. was calculated. Error bars indicate mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.005.
Fig. S3.
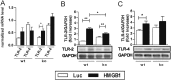
TLRs played an important role in HMGB1-mediated myocardial inflammation. Wt and RAGE-ko mice were treated with AAV9 vectors (HMGB1 or Luc) and immunized only with CFA. Measurements were done on day 21. (A) Myocardial gene expression. (B and C) Protein levels of TLR-2 and -4. Signals from Western blots were normalized against GAPDH. Error bars indicate mean ± SEM. *P < 0.05, **P < 0.01.
Fig. 5.
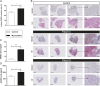
Myocarditis patients displayed increased myocardial expression of HMGB1 and elevated levels of HMGB1 and sRAGE in plasma. (A) The number of nuclear HMGB1-positive cells was increased in the myocardium of patients with myocarditis (n = 13) compared with those of the control group (n = 11). (B) Representative histopathological stainings (HMGB1 and HE) of endomyocardial biopsies (three biopsies for each patient shown). [Scale bars, 500 µm (columns 1–3) and 100 µm (column 4).] (C and D) Patients with myocarditis (n = 10) displayed significantly elevated levels of HMGB1 (C) and sRAGE (D) in their plasma compared with healthy controls (n = 11). Error bars indicate mean ± SEM. *P < 0.05, ***P < 0.005.
Similar articles
- The myocardial infarct-exacerbating effect of cell-free DNA is mediated by the high-mobility group box 1-receptor for advanced glycation end products-Toll-like receptor 9 pathway.
Tian Y, Charles EJ, Yan Z, Wu D, French BA, Kron IL, Yang Z. Tian Y, et al. J Thorac Cardiovasc Surg. 2019 Jun;157(6):2256-2269.e3. doi: 10.1016/j.jtcvs.2018.09.043. Epub 2018 Oct 5. J Thorac Cardiovasc Surg. 2019. PMID: 30401529 Free PMC article. - Expatiating the molecular approaches of HMGB1 in diabetes mellitus: Highlighting signalling pathways via RAGE and TLRs.
Behl T, Sharma E, Sehgal A, Kaur I, Kumar A, Arora R, Pal G, Kakkar M, Kumar R, Bungau S. Behl T, et al. Mol Biol Rep. 2021 Feb;48(2):1869-1881. doi: 10.1007/s11033-020-06130-x. Epub 2021 Jan 21. Mol Biol Rep. 2021. PMID: 33479829 Review. - Targeting high mobility group box protein 1 ameliorates testicular inflammation in experimental autoimmune orchitis.
Aslani F, Schuppe HC, Guazzone VA, Bhushan S, Wahle E, Lochnit G, Lustig L, Meinhardt A, Fijak M. Aslani F, et al. Hum Reprod. 2015 Feb;30(2):417-31. doi: 10.1093/humrep/deu320. Epub 2014 Dec 1. Hum Reprod. 2015. PMID: 25452436 - Blocking the receptor for advanced glycation end product activation attenuates autoimmune myocarditis.
Yang WI, Lee D, Lee DL, Hong SY, Lee SH, Kang SM, Choi DH, Jang Y, Kim SH, Park S. Yang WI, et al. Circ J. 2014;78(5):1197-205. doi: 10.1253/circj.cj-13-1235. Epub 2014 Mar 6. Circ J. 2014. PMID: 24599045 - Clinical Implications of High-mobility Group Box-1 (HMGB1) and the Receptor for Advanced Glycation End-products (RAGE) in Cutaneous Malignancy: A Systematic Review.
Nguyen AH, Detty SQ, Agrawal DK. Nguyen AH, et al. Anticancer Res. 2017 Jan;37(1):1-7. doi: 10.21873/anticanres.11282. Anticancer Res. 2017. PMID: 28011467 Review.
Cited by
- Aging-induced aberrant RAGE/PPARα axis promotes hepatic steatosis via dysfunctional mitochondrial β oxidation.
Wan J, Wu X, Chen H, Xia X, Song X, Chen S, Lu X, Jin J, Su Q, Cai D, Liu B, Li B. Wan J, et al. Aging Cell. 2020 Oct;19(10):e13238. doi: 10.1111/acel.13238. Epub 2020 Sep 16. Aging Cell. 2020. PMID: 32936538 Free PMC article. - Inhibiting Receptor of Advanced Glycation End Products Attenuates Pressure Overload-Induced Cardiac Dysfunction by Preventing Excessive Autophagy.
Gao W, Zhou Z, Liang B, Huang Y, Yang Z, Chen Y, Zhang L, Yan C, Wang J, Lu L, Wen Z, Xian S, Wang L. Gao W, et al. Front Physiol. 2018 Sep 24;9:1333. doi: 10.3389/fphys.2018.01333. eCollection 2018. Front Physiol. 2018. PMID: 30319444 Free PMC article. - Effect of SARS-CoV-2 mRNA-Vaccine on the Induction of Myocarditis in Different Murine Animal Models.
Zirkenbach VA, Ignatz RM, Öttl R, Cehreli Z, Stroikova V, Kaya M, Lehmann LH, Preusch MR, Frey N, Kaya Z. Zirkenbach VA, et al. Int J Mol Sci. 2023 Mar 6;24(5):5011. doi: 10.3390/ijms24055011. Int J Mol Sci. 2023. PMID: 36902442 Free PMC article. - Toll-like receptor 4 in pancreatic damage and immune infiltration in acute pancreatitis.
Mattke J, Darden CM, Lawrence MC, Kuncha J, Shah YA, Kane RR, Naziruddin B. Mattke J, et al. Front Immunol. 2024 Mar 22;15:1362727. doi: 10.3389/fimmu.2024.1362727. eCollection 2024. Front Immunol. 2024. PMID: 38585277 Free PMC article. Review. - HMGB1 Knockout Decreases Kaposi's Sarcoma-Associated Herpesvirus Virion Production in iSLK BAC16 Cells by Attenuating Viral Gene Expression.
Kang SK, Kang YH, Yoo SM, Park C, Kim HS, Lee MS. Kang SK, et al. J Virol. 2021 Jul 26;95(16):e0079921. doi: 10.1128/JVI.00799-21. Epub 2021 Jul 26. J Virol. 2021. PMID: 34105998 Free PMC article.
References
- Göser S, et al. Cardiac troponin I but not cardiac troponin T induces severe autoimmune inflammation in the myocardium. Circulation. 2006;114(16):1693–1702. - PubMed
- Kaya Z, et al. Contribution of the innate immune system to autoimmune myocarditis: A role for complement. Nat Immunol. 2001;2(8):739–745. - PubMed
- Eriksson S, Halenius H, Pulkki K, Hellman J, Pettersson K. Negative interference in cardiac troponin I immunoassays by circulating troponin autoantibodies. Clin Chem. 2005;51(5):839–847. - PubMed
- Leuschner F, et al. Absence of auto-antibodies against cardiac troponin I predicts improvement of left ventricular function after acute myocardial infarction. Eur Heart J. 2008;29(16):1949–1955. - PubMed
- Oozawa S, et al. 2008. Effects of HMGB1 on ischemia-reperfusion injury in the rat heart. Circ J 72(7):1178–1184.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials