Role of Viral RNA and Co-opted Cellular ESCRT-I and ESCRT-III Factors in Formation of Tombusvirus Spherules Harboring the Tombusvirus Replicase - PubMed (original) (raw)
Role of Viral RNA and Co-opted Cellular ESCRT-I and ESCRT-III Factors in Formation of Tombusvirus Spherules Harboring the Tombusvirus Replicase
Nikolay Kovalev et al. J Virol. 2016.
Abstract
Plus-stranded RNA viruses induce membrane deformations in infected cells in order to build viral replication complexes (VRCs). Tomato bushy stunt virus (TBSV) co-opts cellular ESCRT (endosomal sorting complexes required for transport) proteins to induce the formation of vesicle (spherule)-like structures in the peroxisomal membrane with tight openings toward the cytosol. In this study, using a yeast (Saccharomyces cerevisiae) vps23Δ bro1Δ double-deletion mutant, we showed that the Vps23p ESCRT-I protein (Tsg101 in mammals) and Bro1p (ALIX) ESCRT-associated protein, both of which bind to the viral p33 replication protein, play partially complementary roles in TBSV replication in cells and in cell extracts. Dual expression of dominant-negative versions of Arabidopsis homologs of Vps23p and Bro1p inhibited tombusvirus replication to greater extent than individual expression in Nicotiana benthamiana leaves. We also demonstrated the critical role of Snf7p (CHMP4), Vps20p, and Vps24p ESCRT-III proteins in tombusvirus replication in yeast and in vitro. Electron microscopic imaging of vps23Δ yeast revealed the lack of tombusvirus-induced spherule-like structures, while crescent-like structures are formed in ESCRT-III deletion yeasts replicating TBSV RNA. In addition, we also showed that the length of the viral RNA affects the sizes of spherules formed in N. benthamiana cells. The 4.8-kb genomic RNA is needed for the formation of spherules 66 nm in diameter, while spherules formed during the replication of the ∼600-nucleotide (nt)-long defective interfering RNA in the presence of p33 and p92 replication proteins are 42 nm. We propose that the viral RNA serves as a "measuring string" during VRC assembly and spherule formation.
Importance: Plant positive-strand RNA viruses, similarly to animal positive-strand RNA viruses, replicate in membrane-bound viral replicase complexes in the cytoplasm of infected cells. Identification of cellular and viral factors affecting the formation of the membrane-bound viral replication complex is a major frontier in current virology research. In this study, we dissected the functions of co-opted cellular ESCRT-I (endosomal sorting complexes required for transport I) and ESCRT-III proteins and the viral RNA in tombusvirus replicase complex formation using in vitro, yeast-based, and plant-based approaches. Electron microscopic imaging revealed the lack of tombusvirus-induced spherule-like structures in ESCRT-I or ESCRT-III deletion yeasts replicating TBSV RNA, demonstrating the requirement for these co-opted cellular factors in tombusvirus replicase formation. The work could be of broad interest in virology and beyond.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
FIG 1
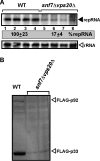
Double deletion of VPS23 ESCRT-I- and BRO1 ESCRT-associated genes inhibits TBSV repRNA accumulation in yeast. (A) The wt, single-deletion _vps23_Δ and _bro1_Δ, or double-deletion _vps23_Δ _bro1_Δ yeast strains were used for these experiments. Top panel, Northern blot analysis of TBSV repRNA accumulation in yeast strains 24 h after induction. The repRNA levels were normalized based on rRNA loading. Bottom panel, Northern blot analysis shows the level of rRNA loading. (B) Detection of FLAG-tagged p33 and FLAG-p92 in yeast mutants supporting TBSV repRNA replication by Western blotting using anti-FLAG antibody. The total protein level in each sample was analyzed by SDS-PAGE and Coomassie blue staining. (C) Detection of FLAG-tagged p33 and FLAG-p92 in yeast mutants in the absence of TBSV repRNA by Western blotting using anti-FLAG antibody.
FIG 2
Expression of dominant-negative mutants of A. thaliana_VPS23_ (At_VPS23_) ESCRT-I and At_BRO1_ (ALIX) ESCRT-associated genes inhibits tombusvirus RNA accumulation in N. benthamiana leaves. Top panel, Northern blot analysis of Cucumber necrosis virus (CNV) (a very close relative of TBSV) RNA accumulation in plants. Individual or dual expression of N-terminal deletion mutants of the two homologous AtVps23p proteins or the N-terminal deletion mutant AtBro1p ESCRT proteins in plants was done in N. benthamiana leaves, which were coinfiltrated with Agrobacterium carrying a plasmid to launch CNV replication from the 35S promoter. The control samples were obtained from leaves expressing no proteins (35S, lanes 1 and 2). Total RNA was extracted from leaves at 2.5 days after agroinfiltration. The accumulation of CNV gRNA and subgenomic (sg) RNAs in N. benthamiana leaves was measured by Northern blotting. Bottom panel, the rRNA was used as a loading control and shown in an agarose gel stained with ethidium bromide, which also allows the detection of the CNV gRNA as the top band. (B) Phenotypes of plants coexpressing dominant-negative mutants of AtVps23p and AtBro1p and symptoms induced by CNV infection. The picture was taken 10 days after agroinfiltration with CNV. (C) Detection of p33 in N. benthamiana leaves coexpressing p92 and TBSV DI-72 RNA (lanes 1 to 6) or p92 (without the TBSV DI-72 RNA) (lanes 7 to 12) and dominant-negative mutants of At_VPS23_ ESCRT-I and At_BRO1_ (ALIX). p33 was detected by Western blotting using anti-p33 antibody. The total protein level in each sample was analyzed by SDS-PAGE and Coomassie blue staining.
FIG 3
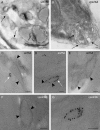
Absence of tombusvirus-induced spherule-like structure formation in _vps23_Δ yeast. (A to C) TEM of stained ultrathin sections of yeast cells replicating TBSV repRNA after staining with uranyl acetate and lead citrate. Elongated and flat bag-like structures in _vps23_Δ yeast expressing p33, p92, and the viral (+)repRNA are indicated by arrows. (D) The TEM image from WT yeast is shown on the right, with white arrows pointing at vesicle-like spherules. (E to G) Distribution of viral dsRNA and p33 replication protein in _vps23_Δ yeast studied by immunogold EM and METTEM, respectively. Ultrathin sections of yeasts expressing MT-p33 and replicating TBSV repRNA are shown. The viral dsRNA (white arrows) was detected with anti-dsRNA antibodies and 10-nm gold particles. MT-p33 is observed via ∼1-nm gold nanoclusters associated with MT (indicated by black arrowheads). Note that the sections were studied without previous staining. We found p33 and the viral dsRNA in the same peripheral compartments. Similar studies with wt yeast have been published previously (see the text). Bars, 100 nm.
FIG 4
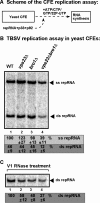
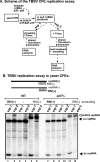
Cell-free TBSV replication assay supports a role for Vps23p and Bro1p in TBSV replication. (A) Scheme of the CFE-based TBSV replication assay. (B) Denaturing PAGE analysis of the 32P-labeled TBSV repRNA products obtained in the CFE-based assay programmed with _in vitro_-transcribed TBSV DI-72 (+)repRNA and purified recombinant MBP-p33 and MBP-p92pol replication proteins of TBSV. The CFEs were prepared from BY4741 or the mutant yeast strains. Each experiment was repeated three times. (C) Increased sensitivity of viral dsRNA products to dsRNA-specific V1 nuclease treatment in the CFE-based TBSV replication assay. We added V1 nuclease at the end of the TBSV replicase assay prior to phenol-chloroform extraction. Nondenaturing PAGE analysis of the 32P-labeled TBSV double-stranded repRNA products obtained in the CFE assays is shown. Each experiment was repeated three times and the data were used to calculate standard deviations.
FIG 5
Double deletion of the SNF7 and VPS20 ESCRT-III genes inhibits TBSV repRNA accumulation in yeast. (A) The wt and the double-deletion _snf7_Δ _vps20_Δ yeast strains were used for these experiments. Top panel, Northern blot analysis of TBSV repRNA accumulation in yeast strains 24 h after induction. The repRNA levels were normalized based on rRNA loading. Bottom panel, Northern blot analysis shows the level of rRNA loading. (B) Detection of FLAG-tagged p33 and FLAG-p92 in the double-deletion _snf7_Δ _vps20_Δ yeast strain by Western blotting using anti-FLAG antibody.
FIG 6
Novel tombusvirus-induced crescent-like structure formation in _snf7_Δ and _vps24_Δ yeast strains. (A and B) TEM of stained ultrathin sections of yeast cells replicating TBSV repRNA after staining with uranyl acetate and lead citrate. The crescent-like structures are indicated by arrows. The crescent-shaped membrane structures with wide openings to the cytosol may represent incomplete spherules that could not constrict the opening due to the lack of the given ESCRT-III factors in the _snf7_Δ and _vps24_Δ (52) yeast strains. Similar studies with wt yeast have been published previously (see the text). (C to G) Distribution of viral dsRNA and p33 replication protein studied by immunogold EM and METTEM, respectively, in _snf7_Δ and _vps24_Δ yeast strains. Ultrathin sections of yeasts expressing MT-p33, p92, and replicating TBSV repRNA are shown. The viral dsRNA (indicated by white arrows) was detected with anti-dsRNA antibodies and 10-nm gold particles. MT-p33 is observed via ∼1-nm gold nanoclusters associated with MT (black arrowheads). Note that the sections were studied without previous staining. We found p33 and the viral dsRNA in the same peripheral compartments. Similar studies with wt yeast have been published previously (see the text). Bars, 100 nm.
FIG 7
Cell-free TBSV replication assay supports a role for Snf7p, Vps20p, and Vps24p ESCRT-III proteins in viral replication. (A) Scheme of the CFE-based TBSV replication assay using CFEs from the _snf7_Δ, _vps20_Δ, _vps24_Δ, and double-deletion _snf7_Δ _vps20_Δ yeast strains. (B) Nondenaturing PAGE analysis of the 32P-labeled TBSV repRNA products obtained in the CFE-based assay programmed with _in vitro_-transcribed TBSV DI-72 (+)repRNA and purified recombinant MBP-p33 and MBP-p92pol replication proteins of TBSV. The newly produced TBSV ssRNA and dsRNA are indicated. The CFEs were prepared from BY4741 or the mutant yeast strains as shown. Note that samples were obtained from the total CFE reactions, except in lanes 10 and 12, where the samples were obtained using only the membrane-fraction of CFE prepared after termination of the in vitro replication assay [thus removing most of the newly made (+)RNAs that are released to the soluble fraction during the assay]. Each experiment was repeated three times, and the data were used to calculate standard deviations.
FIG 8
Asymmetric TBSV RNA replication occurs in the absence of Snf7p in the CFE assay. (A) Scheme of the CFE-based TBSV replication assay to demonstrate the presence of various repRNA products. In experiment 1, we added unlabeled short complementary RNAs to the CFE assay mixture in the presence of 0.1% Triton X-100 prior to phenol-chloroform extraction and RNA analysis at the end of the TBSV replication assay. In experiment 2, we added 0.1% Triton X-100 to the CFE assay mixture, performed phenol-chloroform extraction, heat denatured the RNAs, and then added unlabeled short complementary RNAs at the end of the replication assay. Note that experiment 2 tests if the short complementary RNAs could specifically anneal to the target repRNA in the assay. (B) Top, annealing of the unlabeled short complementary RNAs to the 32P-labeled repRNA products is shown schematically. Note that the annealed RNA duplex changes the migration of the RNA in nondenaturing PAGE. Bottom, representative nondenaturing PAGE analysis of 32P-labeled repRNA products synthesized by the tombusvirus replicase in the CFE assay. The CFEs were prepared from the BY4741 or _snf7_Δ yeast strains as for Fig. 7. The positions of shifted repRNAs, which are due to the annealing to short complementary RNAs, single-stranded repRNAs, and double-stranded repRNAs, are shown. Each experiment was repeated three times.
FIG 9
EM images of spherule-like structures induced by tombusvirus replication in N. benthamiana leaves. (A to C) TEM of stained ultrathin sections of N. benthamiana cells replicating CNV genomic RNA and processed for ultrastructural analysis. Several characteristic membranous compartments with tombusvirus-induced spherules are depicted with black arrowheads, while virion-like structures are indicated with gray arrowheads. Bars, 200 nm. (D and F) TEM of stained ultrathin sections of N. benthamiana cells replicating DI-72 RNA in the presence of CNV p33 and p92 replication proteins, which were expressed via agroinfiltration. Tombusvirus-induced spherules are depicted with black arrowheads. Note that the vesicle-like spherules are approximately half the size of the spherules induced in CNV genomic RNA-replicating cells (see Results). Bars, 100 nm.
FIG 10
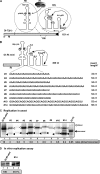
Inefficient replication of short TBSV repRNAs in yeast. (A and B) Schematic representation of TBSV DI-72 (+)repRNA and DI-RI-mini repRNA carrying the three known _cis_-acting replication elements, including the p33 recruitment element (p33RE), the replication silencer element (RSE) required for VRC assembly, and the genomic promoter (gPR) for initiation of (−)RNA synthesis. The characteristic C · C mismatch within p33RE, which is critical for binding of p33/p92 replication proteins, is highlighted. The complementary nucleotides in the RSE and gPR that form a 5-bp-long region are depicted with an arrow. The heterologous sequences of various lengths inserted into DI-RI-mini repRNA are listed. (C) Northern blot analysis of the accumulation of DI-RI-mini repRNA and its insertion derivatives (see panel B) in yeast expressing p33 and p92. The original-size (monomeric) DI-RI-mini repRNA and its derivatives are depicted with black arrowheads, while the double-size (dimeric, head-to-tail fashion) recombinant RNAs are marked with open arrowheads. The ratio of dimer recombination-based versus monomer repRNA in each sample is shown. Note that in comparison with the other samples, the DI-72 repRNA sample was diluted 10 times to avoid overloading. Each experiment was done three times. (D) Representative denaturing gel of 32P-labeled RNA products synthesized by affinity-purified TBSV replicase preparations obtained from yeast replicating either DI-RI-mini repRNA or its derivative (#14 in panel B). The replicase preparations were adjusted to contain comparable levels of p33, and then RI/III(−) template was added to program the in vitro assay.
FIG 11
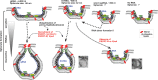
Model of the roles of viral (+)RNA and the co-opted ESCRT proteins during TBSV replication. The formation of tombusvirus-induced spherules (membrane invaginations) in the peroxisomal membranes is facilitated by the recruitment of cellular ESCRT proteins by the mono- or biubiquitinated p33 replication proteins. The model predicts that the size of the recruited viral (+)RNA affects spherule formation by being used as a “measuring string,” possibly by the p33 replication protein. Short repRNAs (∼350 nt) go through a rapid recombination process to generate dimer-sized recombinant RNAs, which are likely more suitable for replication and possibly spherule formation. In the absence of one of the ESCRT-III proteins, the spherule formation is defective and tombusvirus replication proteins could form only membrane invaginations with large openings that make the replicase complex RNase sensitive. Limited TBSV replication might occur between the two crescent-shaped membrane layers in the shown image from the _vps24_Δ yeast strain (bottom right). See the text for further explanation.
Similar articles
- A unique role for the host ESCRT proteins in replication of Tomato bushy stunt virus.
Barajas D, Jiang Y, Nagy PD. Barajas D, et al. PLoS Pathog. 2009 Dec;5(12):e1000705. doi: 10.1371/journal.ppat.1000705. Epub 2009 Dec 24. PLoS Pathog. 2009. PMID: 20041173 Free PMC article. - Co-opted Cellular Sac1 Lipid Phosphatase and PI(4)P Phosphoinositide Are Key Host Factors during the Biogenesis of the Tombusvirus Replication Compartment.
Sasvari Z, Lin W, Inaba JI, Xu K, Kovalev N, Nagy PD. Sasvari Z, et al. J Virol. 2020 Jun 1;94(12):e01979-19. doi: 10.1128/JVI.01979-19. Print 2020 Jun 1. J Virol. 2020. PMID: 32269127 Free PMC article. - The role of co-opted ESCRT proteins and lipid factors in protection of tombusviral double-stranded RNA replication intermediate against reconstituted RNAi in yeast.
Kovalev N, Inaba JI, Li Z, Nagy PD. Kovalev N, et al. PLoS Pathog. 2017 Jul 31;13(7):e1006520. doi: 10.1371/journal.ppat.1006520. eCollection 2017 Jul. PLoS Pathog. 2017. PMID: 28759634 Free PMC article. - Tombusvirus polymerase: Structure and function.
Gunawardene CD, Donaldson LW, White KA. Gunawardene CD, et al. Virus Res. 2017 Apr 15;234:74-86. doi: 10.1016/j.virusres.2017.01.012. Epub 2017 Jan 19. Virus Res. 2017. PMID: 28111194 Review.
Cited by
- Proviral role of ATG2 autophagy related protein in tomato bushy stunt virus replication through bulk phospholipid transfer into the viral replication organelle.
Kang Y, Pogany J, Nagy PD. Kang Y, et al. Mol Biol Cell. 2024 Oct 1;35(10):ar124. doi: 10.1091/mbc.E24-05-0236. Epub 2024 Aug 7. Mol Biol Cell. 2024. PMID: 39110527 - ALIX and TSG101 are essential for cellular entry and replication of two porcine alphacoronaviruses.
Chen X, Liang Y, Weng Z, Hu C, Peng Y, Sun Y, Gao Q, Huang Z, Tang S, Gong L, Zhang G. Chen X, et al. PLoS Pathog. 2024 Mar 15;20(3):e1012103. doi: 10.1371/journal.ppat.1012103. eCollection 2024 Mar. PLoS Pathog. 2024. PMID: 38489378 Free PMC article. - Subversion of selective autophagy for the biogenesis of tombusvirus replication organelles inhibits autophagy.
Kang Y, Lin W, Nagy PD. Kang Y, et al. PLoS Pathog. 2024 Mar 14;20(3):e1012085. doi: 10.1371/journal.ppat.1012085. eCollection 2024 Mar. PLoS Pathog. 2024. PMID: 38484009 Free PMC article. - A critical review on bioaerosols-dispersal of crop pathogenic microorganisms and their impact on crop yield.
Mohaimin AZ, Krishnamoorthy S, Shivanand P. Mohaimin AZ, et al. Braz J Microbiol. 2024 Mar;55(1):587-628. doi: 10.1007/s42770-023-01179-9. Epub 2023 Nov 25. Braz J Microbiol. 2024. PMID: 38001398 Review. - Dynamin-related protein 2 interacts with the membrane-associated methyltransferase domain of plantago asiatica mosaic virus replicase and promotes viral replication.
Shinji H, Sasaki N, Hamim I, Itoh Y, Taku K, Hayashi Y, Minato N, Moriyama H, Arie T, Komatsu K. Shinji H, et al. Virus Res. 2023 Jul 2;331:199128. doi: 10.1016/j.virusres.2023.199128. Epub 2023 May 14. Virus Res. 2023. PMID: 37149224 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous