Defective Leukocyte Adhesion and Chemotaxis Contributes to Combined Immunodeficiency in Humans with Autosomal Recessive MST1 Deficiency - PubMed (original) (raw)
Case Reports
doi: 10.1007/s10875-016-0232-2. Epub 2016 Jan 22.
Joseph D P Willet 1, Helen R Griffin 2, Neil V Morgan 3, Graeme O'Boyle 1, Peter D Arkwright 4, Stephen M Hughes 4, Mario Abinun 1 5, Louise J Tee 3, Dawn Barge 6, Karin R Engelhardt 1, Michael Jackson 5, Andrew J Cant 1 5, Eamonn R Maher 3 7, Mauro Santibanez Koref 2, Louise N Reynard 1, Simi Ali 1, Sophie Hambleton 8 9
Affiliations
- PMID: 26801501
- PMCID: PMC4769310
- DOI: 10.1007/s10875-016-0232-2
Case Reports
Defective Leukocyte Adhesion and Chemotaxis Contributes to Combined Immunodeficiency in Humans with Autosomal Recessive MST1 Deficiency
Tarana Singh Dang et al. J Clin Immunol. 2016 Feb.
Erratum in
- Erratum to: Defective Leukocyte Adhesion and Chemotaxis Contributes to Combined Immunodeficiency in Humans with Autosomal Recessive MST1 Deficiency.
Dang TS, Willet JD, Griffin HR, Morgan NV, O'Boyle G, Arkwright PD, Hughes SM, Abinun M, Tee LJ, Barge D, Engelhardt KR, Jackson M, Cant AJ, Maher ER, Koref MS, Reynard LN, Ali S, Hambleton S. Dang TS, et al. J Clin Immunol. 2016 Apr;36(3):336-7. doi: 10.1007/s10875-016-0248-7. J Clin Immunol. 2016. PMID: 26941167 Free PMC article. No abstract available.
Abstract
Purpose: To investigate the clinical and functional aspects of MST1 (STK4) deficiency in a profoundly CD4-lymphopenic kindred with a novel homozygous nonsense mutation in STK4. Although recent studies have described the cellular effects of murine Mst1 deficiency, the phenotype of MST1-deficient human lymphocytes has yet to be fully explored. Patient lymphocytes were therefore investigated in the context of current knowledge of murine Mst1 deficiency.
Methods: Genetic etiology was identified by whole exome sequencing of genomic DNA from two siblings, combined with linkage analysis in the wider family. MST1 protein expression was assessed by immunoblotting. The ability of patient lymphocytes to adhere to ICAM-1 under flow conditions was measured, and transwell assays were used to assess chemotaxis. Chemokine receptor expression was examined by flow cytometry and receptor signalling by immunoblotting.
Results: A homozygous nonsense mutation in STK4 (c.442C > T, p.Arg148Stop) was found in the patients, leading to a lack of MST1 protein expression. Patient leukocytes exhibited deficient chemotaxis after stimulation with CXCL11, despite preserved expression of CXCR3. Patient lymphocytes were also unable to bind effectively to immobilised ICAM-1 under flow conditions, in keeping with a failure to develop high affinity binding.
Conclusion: The observed abnormalities of adhesion and migration imply a profound trafficking defect among human MST1-deficient lymphocytes. By analogy with murine Mst1 deficiency and other defects of leucocyte trafficking, this is likely to contribute to immunodeficiency by impairing key aspects of T-cell development and function such as positive selection in the thymus, thymic egress and immune synapse formation in the periphery.
Keywords: CD4 lymphopenia; Combined immunodeficiency; MST1; STK4; chemotaxis; lymphocyte adhesion.
Figures
Fig. 1
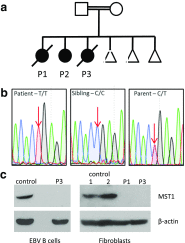
a Pedigree of affected family, demonstrating consanguinity (double horizontal lines). Males and females are indicated by squares and circles respectively, with open symbols indicating healthy individuals and shaded symbols indicating affected individuals. Triangles indicate unaffected individuals with censored gender, and diagonal lines indicate deceased individuals. b Sanger sequencing of STK4 gene confirmed WES findings – a homozygous C to T substitution at position 442 in patients that was heterozygous in both parents. c Western blotting of P3-derived EBV-transformed B cell lysate and P1 and P3 fibroblast lysates, demonstrating MST1 deficiency. Control lysates were derived from random healthy individuals. Representative of a minimum of two separate experiments
Fig. 2
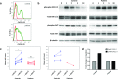
a Patient CD4+ T lymphocytes exhibit expression of the CXCL11 receptor CXCR3 (red histogram = unstained control, green histogram = anti-CXCR3-PE antibody) (n = 1). b EBV-transformed B cells were treated with 12 nM CXCL11 to stimulate CXCR3 before whole cell lysis, Western transfer analysis and probing for phospho-ERK, total ERK, phospho-AKT and total AKT, with β-tubulin as a loading control. Numbers indicate time (mins) of treatment with CXCL11. Representative of three separate experiments. c CXCL11 treated patient EBV-transformed B cells (left panel) show reduced binding to ICAM-1-coated chips under flow conditions when compared to control cells (* indicates P = 0.0313). Patient PBLs also show reduced binding when compared to control cells (right panel). Each shape represents one of six separate experiments, with 5 replicates for each. Significance was determined using Wilcoxon signed rank tests. d CXCL11-treated patient PBLs show unchanged capacity for migration across transwell filters when compared to control cells (n = 1)
Similar articles
- A Novel STK4 Mutation Impairs T Cell Immunity Through Dysregulation of Cytokine-Induced Adhesion and Chemotaxis Genes.
Guennoun A, Bougarn S, Khan T, Mackeh R, Rahman M, Al-Ali F, Ata M, Aamer W, Prosser D, Habib T, Chin-Smith E, Al-Darwish K, Zhang Q, Al-Shakaki A, Robay A, Crystal RG, Fakhro K, Al-Naimi A, Al Maslamani E, Tuffaha A, Janahi I, Janahi M, Love DR, Karim MY, Lo B, Hassan A, Adeli M, Marr N. Guennoun A, et al. J Clin Immunol. 2021 Nov;41(8):1839-1852. doi: 10.1007/s10875-021-01115-2. Epub 2021 Aug 24. J Clin Immunol. 2021. PMID: 34427831 Free PMC article. - EBV Negative Lymphoma and Autoimmune Lymphoproliferative Syndrome Like Phenotype Extend the Clinical Spectrum of Primary Immunodeficiency Caused by STK4 Deficiency.
Schipp C, Schlütermann D, Hönscheid A, Nabhani S, Höll J, Oommen PT, Ginzel S, Fleckenstein B, Stork B, Borkhardt A, Stepensky P, Fischer U. Schipp C, et al. Front Immunol. 2018 Oct 16;9:2400. doi: 10.3389/fimmu.2018.02400. eCollection 2018. Front Immunol. 2018. PMID: 30386345 Free PMC article. - The phenotype of human STK4 deficiency.
Abdollahpour H, Appaswamy G, Kotlarz D, Diestelhorst J, Beier R, Schäffer AA, Gertz EM, Schambach A, Kreipe HH, Pfeifer D, Engelhardt KR, Rezaei N, Grimbacher B, Lohrmann S, Sherkat R, Klein C. Abdollahpour H, et al. Blood. 2012 Apr 12;119(15):3450-7. doi: 10.1182/blood-2011-09-378158. Epub 2012 Jan 31. Blood. 2012. PMID: 22294732 Free PMC article. - MST1/2 Balance Immune Activation and Tolerance by Orchestrating Adhesion, Transcription, and Organelle Dynamics in Lymphocytes.
Ueda Y, Kondo N, Kinashi T. Ueda Y, et al. Front Immunol. 2020 May 6;11:733. doi: 10.3389/fimmu.2020.00733. eCollection 2020. Front Immunol. 2020. PMID: 32435241 Free PMC article. Review. - Diversity in Serine/Threonine Protein Kinase-4 Deficiency and Review of the Literature.
Cagdas D, Halacli SO, Tan C, Esenboga S, Karaatmaca B, Cetinkaya PG, Balcı-Hayta B, Ayhan A, Uner A, Orhan D, Boztug K, Ozen S, Topaloglu R, Sanal O, Tezcan I. Cagdas D, et al. J Allergy Clin Immunol Pract. 2021 Oct;9(10):3752-3766.e4. doi: 10.1016/j.jaip.2021.05.032. Epub 2021 Jun 17. J Allergy Clin Immunol Pract. 2021. PMID: 34146746 Review.
Cited by
- Primary and Acquired Immunodeficiencies Associated With Severe Varicella-Zoster Virus Infections.
Ansari R, Rosen LB, Lisco A, Gilden D, Holland SM, Zerbe CS, Bonomo RA, Cohen JI. Ansari R, et al. Clin Infect Dis. 2021 Nov 2;73(9):e2705-e2712. doi: 10.1093/cid/ciaa1274. Clin Infect Dis. 2021. PMID: 32856043 Free PMC article. Review. - Human genetic dissection of papillomavirus-driven diseases: new insight into their pathogenesis.
Béziat V. Béziat V. Hum Genet. 2020 Jun;139(6-7):919-939. doi: 10.1007/s00439-020-02183-x. Epub 2020 May 20. Hum Genet. 2020. PMID: 32435828 Free PMC article. Review. - Mammalian sterile 20 kinase 1 and 2 are important regulators of hematopoietic stem cells in stress condition.
Lee DH, Kim TS, Lee D, Lim DS. Lee DH, et al. Sci Rep. 2018 Jan 17;8(1):942. doi: 10.1038/s41598-018-19637-y. Sci Rep. 2018. PMID: 29343826 Free PMC article. - A Novel STK4 Mutation Presenting with Juvenile Idiopathic Arthritis and Epidermodysplasia Verruciformis.
Sharafian S, Ziaee V, Shahrooei M, Ahadi M, Parvaneh N. Sharafian S, et al. J Clin Immunol. 2019 Jan;39(1):11-14. doi: 10.1007/s10875-018-0586-8. Epub 2019 Jan 5. J Clin Immunol. 2019. PMID: 30612220 No abstract available. - Hippo Pathway in Mammalian Adaptive Immune System.
Yamauchi T, Moroishi T. Yamauchi T, et al. Cells. 2019 Apr 30;8(5):398. doi: 10.3390/cells8050398. Cells. 2019. PMID: 31052239 Free PMC article. Review.
References
- Lee KK, Ohyama T, Yajima N, Tsubuki S, Yonehara S. MST, a physiological caspase substrate, highly sensitizes apoptosis both upstream and downstream of caspase activation. The Journal of Biological cHemistry. 2001 Jun 1;276(22):19276–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous