Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer - PubMed (original) (raw)
Clinical Trial
. 2016 Jun 15;22(12):2848-54.
doi: 10.1158/1078-0432.CCR-15-2010. Epub 2016 Jan 26.
William P Harris 2, J Thaddeus Beck 3, Boris A Berdov 4, Stephanie A Wagner 5, Eduard M Pshevlotsky 6, Sergei A Tjulandin 7, Oleg A Gladkov 8, Randall F Holcombe 9, Ronald Korn 10, Natarajan Raghunand 11, Samuel Dychter 12, Ping Jiang 12, H Michael Shepard 12, Craig E Devoe 13
Affiliations
- PMID: 26813359
- PMCID: PMC7787348
- DOI: 10.1158/1078-0432.CCR-15-2010
Clinical Trial
Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer
Sunil R Hingorani et al. Clin Cancer Res. 2016.
Abstract
Purpose: This phase Ib study evaluated the safety and tolerability of PEGylated human recombinant hyaluronidase (PEGPH20) in combination with gemcitabine (Gem), and established a phase II dose for patients with untreated stage IV metastatic pancreatic ductal adenocarcinoma (PDA). Objective response rate and treatment efficacy using biomarker and imaging measurements were also evaluated.
Experimental design: Patients received escalating intravenous doses of PEGPH20 in combination with Gem using a standard 3+3 dose-escalation design. In cycle 1 (8 weeks), PEGPH20 was administrated twice weekly for 4 weeks, then once weekly for 3 weeks; Gem was administrated once weekly for 7 weeks, followed by 1 week off treatment. In each subsequent 4-week cycle, PEGPH20 and Gem were administered once weekly for 3 weeks, followed by 1 week off. Dexamethasone (8 mg) was given pre- and post-PEGPH20 administration. Several safety parameters were evaluated.
Results: Twenty-eight patients were enrolled and received PEGPH20 at 1.0 (n = 4), 1.6 (n = 4), or 3.0 μg/kg (n = 20), respectively. The most common PEGPH20-related adverse events were musculoskeletal and extremity pain, peripheral edema, and fatigue. The incidence of thromboembolic events was 29%. Median progression-free survival (PFS) and overall survival (OS) rates were 5.0 and 6.6 months, respectively. In 17 patients evaluated for pretreatment tissue hyaluronan (HA) levels, median PFS and OS rates were 7.2 and 13.0 months for "high"-HA patients (n = 6), and 3.5 and 5.7 months for "low"-HA patients (n = 11), respectively.
Conclusions: PEGPH20 in combination with Gem was well tolerated and may have therapeutic benefit in patients with advanced PDA, especially in those with high HA tumors. Clin Cancer Res; 22(12); 2848-54. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
S.R. Hingorani is a consultant/advisory board member for Halozyme Therapeutics. W.P. Harris reports receiving a commercial research grant from Halozyme. S. Tjulandin reports receiving speakers bureau honoraria from AstraZeneca and Pfizer. R. Korn has ownership interest (including patents) in Imaging Endpoints. H.M. Shepard reports receiving a commercial research grant from Halozyme and has ownership interest (including patents) in Halozyme. No potential conflicts of interest were disclosed by the other authors.
Figures
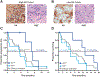
Figure 1.
Representative micrographs of tumor biopsies with high HA (A) and low HA (B) content. HA is detected with HA binding protein, and nuclei are counterstained with hematoxylin. Corresponding hematoxylin and eosin (H&E) stains are also shown. PFS (C) and OS (D) of patients who received either 1.6 or 3.0 μg/kg of PEGPH20+Gem. Gray line, all patients in the efficacy-evaluable population (n = 24); dark blue line, high HA (n = 6, 1 patient withdrew consent and was censored for PFS and OS, 1 patient withdrew consent and was censored for PFS); light blue line, low HA (n = 11, 1 patient who was censored for PFS and OS was withdrawn from study by investigator decision). Magnification, ×200.
Similar articles
- Randomized Phase III Trial of Pegvorhyaluronidase Alfa With Nab-Paclitaxel Plus Gemcitabine for Patients With Hyaluronan-High Metastatic Pancreatic Adenocarcinoma.
Van Cutsem E, Tempero MA, Sigal D, Oh DY, Fazio N, Macarulla T, Hitre E, Hammel P, Hendifar AE, Bates SE, Li CP, Hingorani SR, de la Fouchardiere C, Kasi A, Heinemann V, Maraveyas A, Bahary N, Layos L, Sahai V, Zheng L, Lacy J, Park JO, Portales F, Oberstein P, Wu W, Chondros D, Bullock AJ; HALO 109-301 Investigators. Van Cutsem E, et al. J Clin Oncol. 2020 Sep 20;38(27):3185-3194. doi: 10.1200/JCO.20.00590. Epub 2020 Jul 24. J Clin Oncol. 2020. PMID: 32706635 Free PMC article. Clinical Trial. - HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma.
Hingorani SR, Zheng L, Bullock AJ, Seery TE, Harris WP, Sigal DS, Braiteh F, Ritch PS, Zalupski MM, Bahary N, Oberstein PE, Wang-Gillam A, Wu W, Chondros D, Jiang P, Khelifa S, Pu J, Aldrich C, Hendifar AE. Hingorani SR, et al. J Clin Oncol. 2018 Feb 1;36(4):359-366. doi: 10.1200/JCO.2017.74.9564. Epub 2017 Dec 12. J Clin Oncol. 2018. PMID: 29232172 Clinical Trial. - Phase IB/II Randomized Study of FOLFIRINOX Plus Pegylated Recombinant Human Hyaluronidase Versus FOLFIRINOX Alone in Patients With Metastatic Pancreatic Adenocarcinoma: SWOG S1313.
Ramanathan RK, McDonough SL, Philip PA, Hingorani SR, Lacy J, Kortmansky JS, Thumar J, Chiorean EG, Shields AF, Behl D, Mehan PT, Gaur R, Seery T, Guthrie KA, Hochster HS. Ramanathan RK, et al. J Clin Oncol. 2019 May 1;37(13):1062-1069. doi: 10.1200/JCO.18.01295. Epub 2019 Feb 28. J Clin Oncol. 2019. PMID: 30817250 Free PMC article. Clinical Trial. - Targeting the Tumor Stroma: the Biology and Clinical Development of Pegylated Recombinant Human Hyaluronidase (PEGPH20).
Wong KM, Horton KJ, Coveler AL, Hingorani SR, Harris WP. Wong KM, et al. Curr Oncol Rep. 2017 Jul;19(7):47. doi: 10.1007/s11912-017-0608-3. Curr Oncol Rep. 2017. PMID: 28589527 Review. - Gemcitabine-based combination therapy compared with gemcitabine alone for advanced pancreatic cancer: a meta-analysis of nine randomized controlled trials.
Jin SF, Fan ZK, Pan L, Jin LM. Jin SF, et al. Hepatobiliary Pancreat Dis Int. 2017 Jun;16(3):236-244. doi: 10.1016/s1499-3872(17)60022-5. Hepatobiliary Pancreat Dis Int. 2017. PMID: 28603091 Review.
Cited by
- Brachytherapy via a depot of biopolymer-bound 131I synergizes with nanoparticle paclitaxel in therapy-resistant pancreatic tumours.
Schaal JL, Bhattacharyya J, Brownstein J, Strickland KC, Kelly G, Saha S, Milligan J, Banskota S, Li X, Liu W, Kirsch DG, Zalutsky MR, Chilkoti A. Schaal JL, et al. Nat Biomed Eng. 2022 Oct;6(10):1148-1166. doi: 10.1038/s41551-022-00949-4. Epub 2022 Oct 19. Nat Biomed Eng. 2022. PMID: 36261625 Free PMC article. - Interstitial Pressure in Pancreatic Ductal Adenocarcinoma Is Dominated by a Gel-Fluid Phase.
DuFort CC, DelGiorno KE, Carlson MA, Osgood RJ, Zhao C, Huang Z, Thompson CB, Connor RJ, Thanos CD, Scott Brockenbrough J, Provenzano PP, Frost GI, Michael Shepard H, Hingorani SR. DuFort CC, et al. Biophys J. 2016 May 10;110(9):2106-19. doi: 10.1016/j.bpj.2016.03.040. Biophys J. 2016. PMID: 27166818 Free PMC article. - Stromal dynamic reciprocity in cancer: intricacies of fibroblastic-ECM interactions.
Alexander J, Cukierman E. Alexander J, et al. Curr Opin Cell Biol. 2016 Oct;42:80-93. doi: 10.1016/j.ceb.2016.05.002. Epub 2016 May 20. Curr Opin Cell Biol. 2016. PMID: 27214794 Free PMC article. Review. - Dietary fat stimulates pancreatic cancer growth and promotes fibrosis of the tumor microenvironment through the cholecystokinin receptor.
Nadella S, Burks J, Al-Sabban A, Inyang G, Wang J, Tucker RD, Zamanis ME, Bukowski W, Shivapurkar N, Smith JP. Nadella S, et al. Am J Physiol Gastrointest Liver Physiol. 2018 Nov 1;315(5):G699-G712. doi: 10.1152/ajpgi.00123.2018. Epub 2018 Jun 21. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 29927319 Free PMC article. - Modulating the Tumor Microenvironment to Enhance Tumor Nanomedicine Delivery.
Zhang B, Hu Y, Pang Z. Zhang B, et al. Front Pharmacol. 2017 Dec 22;8:952. doi: 10.3389/fphar.2017.00952. eCollection 2017. Front Pharmacol. 2017. PMID: 29311946 Free PMC article. Review.
References
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–21. - PubMed
- Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403–13. - PubMed
- Arshad A, Al-Leswas D, Al-Taan O, Stephenson J, Metcalfe M, Steward WP, et al. Pooled survival and response data from phase III randomized controlled trials for gemcitabine-based regimes in the treatment of advanced pancreatic cancer. Am J Clin Oncol 2013;36:411–4. - PubMed
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials