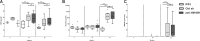Treatment with Anti-HMGB1 Monoclonal Antibody Does Not Affect Lupus Nephritis in MRL/lpr Mice - PubMed (original) (raw)
Treatment with Anti-HMGB1 Monoclonal Antibody Does Not Affect Lupus Nephritis in MRL/lpr Mice
Fleur Schaper et al. Mol Med. 2016 Sep.
Abstract
High mobility group box 1 (HMGB1) is a nuclear DNA binding protein that acts as an alarmin when secreted. HMGB1 is increased in systemic lupus erythematosus and might represent a potential therapeutic target. We investigated whether treatment with an anti-HMGB1 antibody affects the development of lupus nephritis in MRL/lpr mice. Seven-week-old MRL/lpr mice were injected intraperitoneally twice weekly for 10 wks with 50 μg monoclonal anti-HMGB1 (2G7, mouse IgG2b) (n = 12) or control antibody (n = 11). Control MRL/MPJ mice (n = 10) were left untreated. Every 2 wks, blood was drawn and urine was collected at wk 7, 11 and 17. Mice were sacrificed at 17 wks for complete disease evaluation. Plasma HMGB1 and anti-HMGB1 levels were increased in MRL/lpr mice compared with control MRL/MPJ mice. There were no differences in albuminuria, urine HMGB1 and plasma levels of complement C3, anti-dsDNA and proinflammatory cytokines between untreated and treated mice at any time point. Lupus nephritis of mice treated with anti-HMGB1 monoclonal antibody (mAb) was classified as class III (n = 3) and class IV (n = 9), while mice treated with control mAb were classified as class II (n = 4), class III (n = 2) and class IV (n = 5). IgG and C3 deposits in kidneys were similar in mice treated with anti-HMGB1 mAb or control mAb. In conclusion, treatment with monoclonal anti-HMGB-1 antibody 2G7 does not affect development of lupus nephritis, disease progression or proinflammatory cytokine levels in MRL/lpr mice. This result indicates that blocking of HMGB1 by this neutralizing antibody does not affect lupus nephritis in MRL/lpr mice.
Conflict of interest statement
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Figures
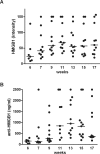
Figure 1
Plasma HMGB1 and anti-HMGB1 antibody levels increase with age in MRL/lpr mice. (A) Plasma HMGB1 levels were measured by Western blot at wks 6, 7, 9, 11, 13, 15 and 17 in MRL/lpr mice (n = 11). HMGB1 levels are presented as values of fluorescence intensity and were normalized against a standard of Jurkat cell lysate. (B) Plasma anti-HMGB levels (ng/mL) were measured by ELISA at wks 6, 7, 9, 11, 13, 15 and 17 in MRL/lpr mice (n = 11). Anti-HMGB1 levels are presented as values of fluorescence intensity and were normalized against a standard of monoclonal anti-HMGB1 antibody.
Figure 2
Anti-HMGB1 mAb treatment of MRL/lpr mice does not affect plasma levels of HMGB1, anti-dsDNA antibodies and complement C3, while increasing HMGB1 antibody levels. (A) Plasma anti-dsDNA (units/mL) was measured by ELISA in MRL/MPJ (n = 10) and MRL/lpr (n = 10–12) mice at wks 7, 9, 13 and 17. (B) Plasma complement C3 (mg/mL) was measured by ELISA in MRL/MPJ (n = 10) and MRL/lpr (n = 10–12) mice at wk 7, 9, 13 and 17. (C) Plasma HMGB1 was measured by Western blotting in MRL/MPJ (n = 10) and MRL/lpr (n = 10–12) mice at wks 7, 9, 11, 13, 15 and 17. HMGB1 levels are presented as values of fluorescence intensity and were normalized against a standard of Jurkat cell lysate. (D) Plasma anti-HMGB1 (ng/mL) was measured by ELISA in MRL/MPJ (n = 10) and MRL/lpr (n = 10–12) mice at wks 7, 9, 11, 13, 15 and 17. Anti-HMGB1 levels are presented as values of fluorescence intensity and were normalized against a standard of monoclonal anti-HMGB1 antibody. Depicted are the median values with range at each time point.
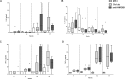
Figure 3
Anti-HMGB1 mAb treatment of MRL/lpr mice does not affect plasma levels of proinflammatory cytokines. Plasma levels of the cytokines IL-6 (A), IL-17A (B), IFN-α (C) and TNF-α (D) were measured by multiplex in MRL/MPJ and MRL/lpr mice at wks 7, 9, 13 and 17. Box and whiskers plot, median and interquartile range are shown for n = 6–8 mice per group. Separate dots indicate outliers.
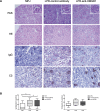
Figure 4
Anti-HMGB1 mAb treatment of MRL/lpr mice does not affect renal pathology and renal immune complex deposition. (A) Representative pictures of PAS, H&E, C3 and IgG staining in kidney sections of 17-wk-old MRL/MPJ and MRL/lpr mice (10×). Inserts show a glomerulus in detail (40×). (B) Quantitative analysis of C3 and IgG deposition in kidney sections of 17-wk-old MRL/MPJ and MRL/lpr mice. Box and whiskers plot, median and interquartile range are shown for n = 8–11 mice per group. A Kruskal-Wallis test was used to test for overall differences between the three groups. To investigate which group was different, further testing was performed to compare groups separately using a Mann-Whitney U test. *p < 0.05
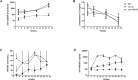
Figure 5
HMGB1 mAb treatment of MRL/lpr mice does not affect albuminuria and HMGB1 urine levels. (A) Renal function was determined by measuring BUN levels at wks 7, 11 and 17. (B) Albumin was measured by ELISA in 18-h urine of MRL/MPJ (n = 10) and MRL/lpr (n = 10–12) mice at wks 7, 11 and 17. (C) HMGB1 was measured by Western blotting in 18-h urine of MRL/MPJ (n = 10) and MRL/lpr (n = 10–12) mice at wks 7, 11 and 17. HMGB1 levels are presented as values of fluorescence intensity and were normalized against a standard of Jurkat cell lysate. A Kruskal-Wallis test was used to test for overall differences between the three groups at each time point. To investigate which group was different, further testing was performed to compare groups separately using a Mann-Whitney U test. Box and whiskers plot, median and interquartile range are shown for n = 8–12 mice per group. Separate dots indicate outliers. ***p < 0.0001. ns, Nonsignificant.
Figure 6
Anti-HMGB1mAb treatment of MRL/lpr mice does not affect renal mRNA levels of IL-6, TNF, MCP-1, NGAL or KIM-1. Expression levels of TNF-α (A), IL-6 (B), MCP-1 (C), NGAL (D) and KIM-1 (E) mRNA were analyzed in kidney tissues of MRL/MPJ mice (n = 8) and MRL/lpr mice treated with control (n = 11) and mice treated with monoclonal anti-HMGB1 (n = 9). Data are shown as relative expression compared with GAPDH. Box and whiskers plot, median and interquartile range are shown. Separate dots indicate outliers. A Kruskal-Wallis test was used to test for overall differences between the three groups. To investigate which group was different, further testing was performed to compare groups separately using a Mann-Whitney U test. *p < 0.05; **p < 0.01. ns, Nonsignificant.
Similar articles
- Anti-high Mobility Group Box 1 Antibody Ameliorates Albuminuria in MRL/lpr Lupus-Prone Mice.
Watanabe H, Watanabe KS, Liu K, Hiramatsu S, Zeggar S, Katsuyama E, Tatebe N, Akahoshi A, Takenaka F, Hanada T, Akehi M, Sasaki T, Sada KE, Matsuura E, Nishibori M, Wada J. Watanabe H, et al. Mol Ther Methods Clin Dev. 2017 May 25;6:31-39. doi: 10.1016/j.omtm.2017.05.006. eCollection 2017 Sep 15. Mol Ther Methods Clin Dev. 2017. PMID: 28649578 Free PMC article. - The new complement inhibitor CRIg/FH ameliorates lupus nephritis in lupus-prone MRL/lpr mice.
Shi Y, Yao W, Sun L, Li G, Liu H, Ding P, Hu W, Xu H. Shi Y, et al. BMC Nephrol. 2019 Nov 21;20(1):424. doi: 10.1186/s12882-019-1599-0. BMC Nephrol. 2019. PMID: 31752725 Free PMC article. - 1,25-dihydroxyvitamin D3 ameliorates lupus nephritis through inhibiting the NF-κB and MAPK signalling pathways in MRL/lpr mice.
Li X, Liu J, Zhao Y, Xu N, Lv E, Ci C, Li X. Li X, et al. BMC Nephrol. 2022 Jul 8;23(1):243. doi: 10.1186/s12882-022-02870-z. BMC Nephrol. 2022. PMID: 35804318 Free PMC article. - Activated protein C attenuates systemic lupus erythematosus and lupus nephritis in MRL-Fas(lpr) mice.
Lichtnekert J, Rupanagudi KV, Kulkarni OP, Darisipudi MN, Allam R, Anders HJ. Lichtnekert J, et al. J Immunol. 2011 Sep 15;187(6):3413-21. doi: 10.4049/jimmunol.1101125. Epub 2011 Aug 17. J Immunol. 2011. PMID: 21849682 - Anti-high mobility group box protein 1 monoclonal antibody downregulating P-glycoprotein as novel epilepsy therapeutics.
de Liyis BG, Tandy SG, Endira JF, Putri KA, Utami DKI. de Liyis BG, et al. Egypt J Neurol Psychiatr Neurosurg. 2022;58(1):121. doi: 10.1186/s41983-022-00557-8. Epub 2022 Oct 22. Egypt J Neurol Psychiatr Neurosurg. 2022. PMID: 36310854 Free PMC article. Review.
Cited by
- Targeting HMGB1 by ethyl pyruvate ameliorates systemic lupus erythematosus and reverses the senescent phenotype of bone marrow-mesenchymal stem cells.
Ji J, Fu T, Dong C, Zhu W, Yang J, Kong X, Zhang Z, Bao Y, Zhao R, Ge X, Sha X, Lu Z, Li J, Gu Z. Ji J, et al. Aging (Albany NY). 2019 Jul 14;11(13):4338-4353. doi: 10.18632/aging.102052. Aging (Albany NY). 2019. PMID: 31303606 Free PMC article. - Anti-high Mobility Group Box 1 Antibody Ameliorates Albuminuria in MRL/lpr Lupus-Prone Mice.
Watanabe H, Watanabe KS, Liu K, Hiramatsu S, Zeggar S, Katsuyama E, Tatebe N, Akahoshi A, Takenaka F, Hanada T, Akehi M, Sasaki T, Sada KE, Matsuura E, Nishibori M, Wada J. Watanabe H, et al. Mol Ther Methods Clin Dev. 2017 May 25;6:31-39. doi: 10.1016/j.omtm.2017.05.006. eCollection 2017 Sep 15. Mol Ther Methods Clin Dev. 2017. PMID: 28649578 Free PMC article. - The multifunctional protein HMGB1: 50 years of discovery.
Tang D, Kang R, Zeh HJ, Lotze MT. Tang D, et al. Nat Rev Immunol. 2023 Dec;23(12):824-841. doi: 10.1038/s41577-023-00894-6. Epub 2023 Jun 15. Nat Rev Immunol. 2023. PMID: 37322174 Review. - Targeting Inflammation Driven by HMGB1.
Yang H, Wang H, Andersson U. Yang H, et al. Front Immunol. 2020 Mar 20;11:484. doi: 10.3389/fimmu.2020.00484. eCollection 2020. Front Immunol. 2020. PMID: 32265930 Free PMC article. Review. - HMGB1 in Systemic Lupus Erythematosus.
Liu T, Son M, Diamond B. Liu T, et al. Front Immunol. 2020 May 27;11:1057. doi: 10.3389/fimmu.2020.01057. eCollection 2020. Front Immunol. 2020. PMID: 32536928 Free PMC article. Review.
References
- Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet. 2014;384:1878–88. - PubMed
- Bianchi ME, Agresti A. HMG proteins: dynamic players in gene regulation and differentiation. Curr. Opin. Genet. Dev. 2005;15:496–506. - PubMed
- Taniguchi N, et al. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 2003;48:971–81. - PubMed
- Kokkola R, et al. High mobility group box chromosomal protein 1: a novel proinflammatory mediator in synovitis. Arthritis Rheum. 2002;46:2598–603. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous