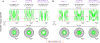Super-resolution 3D tomography of interactions and competition in the nuclear pore complex - PubMed (original) (raw)
Super-resolution 3D tomography of interactions and competition in the nuclear pore complex
Jiong Ma et al. Nat Struct Mol Biol. 2016 Mar.
Abstract
A selective barrier formed by intrinsically disordered Phe-Gly (FG) nucleoporins (Nups) allows transport receptor (TR)-facilitated translocation of signal-dependent cargos through the nuclear pore complexes (NPCs) of eukaryotic cells. However, the configuration of the FG-Nup barrier and its interactions with multiple TRs in native NPCs remain obscure. Here, we mapped the interaction sites of various TRs or FG segments within the FG-Nup barrier by using high-speed super-resolution microscopy and used these sites to reconstruct the three-dimensional tomography of the native barrier in the NPC. We found that each TR possesses a unique interaction zone within the FG-Nup barrier and that two major TRs, importin β1 and Crm1, outcompete other TRs in binding FG Nups. Moreover, TRs may alter the tomography of the FG-Nup barrier and affect one another's pathways under circumstances of heavy competition.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Figures
Figure 1
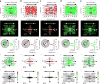
FG-Nup barrier models. (a) Top, the oily-spaghetti model of the FG-Nup barrier in the NPC, shown in side and top views. FG domains (blue lines) form an entropic barrier through which signal-independent small molecules freely diffuse via a central axial channel, and transport receptors (TRs) carry their cargos, interacting with FG Nups (TR-FG interactions), thereby mediating transport through the NPC. Bottom, side and top views of the selective-phase or hydrogel-meshwork model. Hydrophobic interactions among FG repeats (FG-FG interactions) generate a sieve-like hydrogel meshwork (blue mesh), thus allowing signal-independent small molecules to pass through the gaps of the meshwork and TR–cargo complexes and to dissolve into the meshwork through TR-FG interactions. C, cytoplasmic side of the NPC; NE, nuclear envelope; N, nucleoplasmic side of the NPC. (b) FG-FG or TR-FG interactions recognized by fluorescent FG-segment or TR probes, respectively. Reconstruction of these interactions in 3D leads to the tomography of FG-Nup barrier in the NPC. (c) Typical single-molecule snapshots of dwelling positions of the fluorescent probes in the NPC, determined by SPEED microscopy. Dwelling positions (from frames 1 to n) of individual fluorescent probe molecules (red spots) inside a single GFP-labeled NPC (green spot) were fitted by 2D Gaussian fittings. The spatial locations of many such events were superimposed to form a 2D super-resolution image.
Figure 2
Detection of FG-FG and TR-FG interaction sites in the FG-Nup barrier by SPEED microscopy. (a–e) 2D and 3D spatial distributions of WT yNup116 (345–458) (a), charged yNup116(345–458) (b), 10-kDa dextran (c), Imp β1(1–861) (d) and Imp β1(331–861) (e) inside the NPCs of permeabilized cells. Typically, 3,000 to 6,000 spatial locations for each substrate were collected from 15 NPCs of 15 cells. (i) 2D spatial localizations of single molecules located primarily within a rectangular area of 240 × 160 nm around the centroid of the NPC, superimposed on the NPC architecture (gray). (ii) 3D probability density map obtained with a 2D-to-3D transform algorithm (green cloud; brighter color indicates higher density) and shown in 3D cut-away side-view, superimposed on the NPC architecture (gray). N, nucleoplasmic side of the NPC. C, cytoplasmic side of the NPC. (iii) Central lateral slice (top view) at the NPC middle plane of the 3D probability density maps. (iv) Central axial slice (side view) along the nucleocytoplasmic axis of the 3D probability density maps. The ranges of strong (blue dashed squares)- and weak (red dashed ovals)-interaction zones are highlighted and numbered in nanometers. (v) Heat map of iv. Red represents the highest density and black the lowest density in the color scale. A.u., absorbance units; X, r and θ, cylindrical coordinates; H, high; L, low.
Figure 3
3D probability density maps of FG repeats in the FG-Nup barrier recognized by various FG segments, importins and exportins in permeabilized cells. (a–i) Side views of the central axial slices along the nucleocytoplasmic axis of the 3D probability density maps of various FG segments (a–c), importins (d–f) and exportins (g–i). The strong (blue dashed squares)- and weak (red dashed ovals)-interaction zones are highlighted. N, nucleoplasmic side. C, cytoplasmic side. (j) 3/4th cutaway side view of 3D probability density maps of all recognized FG-FG interaction sites from the tested FG segments (green), superimposed on the passive-diffusion pathway of yNup159(441–881) (red) and the NPC architecture (gray). (k) Heat map of the central slice of j. (l,m) Cutaway 3D view (l) and heat map (m) of the probability density map for the importin-recognized FG repeats (green), superimposed on the passive-diffusion pathway of 10-kDa dextran (red) and the NPC architecture (gray). (n,o) Cutaway 3D view (n) and heat map (o) of the probability density map for the exportin-recognized FG repeats (green), superimposed on the passive-diffusion pathway of 10-kDa dextran (red) and the NPC architecture (gray). (p–s) Merged 3D probability density maps and heat maps of the FG repeats in the NPC from j, l and n in both side view (p,q) and top view (r,s). Red represents the highest density and black the lowest density in the color scale. Each of the above measurements was repeated at least three times.
Figure 4
Competition among importins, determined in the NPCs of permeabilized cells. (a) Imp β1’s competition effect on Imp β2. Central-slice side view (top) and top view (bottom) indicating the 3D spatial probability maps of Imp β2 alone (left), Imp β2 in the presence of 15 μM Imp β1 (middle) and Imp β1 in the presence of 15 μM Imp β1 (right). NPC architecture is superimposed in gray. The major competition zone between Imp β1 and Imp β2 is highlighted and numbered in nanometers (red dashed rectangle). (b) Imp β2’s competition effect on Imp β1. (c) Imp β1’s competition effect on NTF2. (d) NTF2’s competition effect on Imp β1. Each of the above measurements was repeated at least three times.
Figure 5
Competition among exportins, determined in the NPCs of permeabilized cells. (a) Crm1’s competition effect on CAS. (b) CAS’s competition effect on Crm1. (c) Crm1’s competition effect on Tap–p15. (d) Tap–p15’s competition effect on Crm1. Figure layout is as described in Figure 4. Each measurement was repeated at least three times.
Figure 6
Competition between importin and exportin during binding to FG Nups in the NPCs of permeabilized cells. (a) Imp β1’s competition effect on Crm1. (b) Crm1’s competition effect on Imp β1. Figure layout is as described in Figure 4. The measurements were repeated at least three times.
Figure 7
3D tomography of interaction sites for Imp β1 and Crm1 in the NPCs of live cells. (a,b) 3D probability density map of GFP-Imp β1 (a) or GFP-Crm1 (b) (green), superimposed on the NPC architecture (gray) in live cells. (i) 3/4th cutaway side view. (ii) Central _xz_-slice view along the nucleocytoplasmic axis (y = 0). The ranges of strong (blue dashed squares)- and weak (red dashed ovals)-interaction zones are highlighted and labeled in nanometers. (iii) Heat map of ii. (iv) Central _yz_-slice view (x = 0) at the NPC middle plane. (v) Heat map of iv. Red represents the highest density and black the lowest density in the color scale. N, nucleoplasmic side of the NPC. C, cytoplasmic side of the NPC. The above measurements were repeated at least three times.
Comment in
- Reply to 'Deconstructing transport-distribution reconstruction in the nuclear-pore complex'.
Ruba A, Kelich J, Ma J, Yang W. Ruba A, et al. Nat Struct Mol Biol. 2018 Dec;25(12):1062-1064. doi: 10.1038/s41594-018-0162-1. Epub 2018 Dec 5. Nat Struct Mol Biol. 2018. PMID: 30518846 Free PMC article. No abstract available. - Deconstructing transport-distribution reconstruction in the nuclear-pore complex.
Tu LC, Huisman M, Chung YC, Smith CS, Grunwald D. Tu LC, et al. Nat Struct Mol Biol. 2018 Dec;25(12):1061-1062. doi: 10.1038/s41594-018-0161-2. Epub 2018 Dec 5. Nat Struct Mol Biol. 2018. PMID: 30518848 Free PMC article.
Similar articles
- The selective permeability barrier in the nuclear pore complex.
Li C, Goryaynov A, Yang W. Li C, et al. Nucleus. 2016 Sep 2;7(5):430-446. doi: 10.1080/19491034.2016.1238997. Epub 2016 Sep 27. Nucleus. 2016. PMID: 27673359 Free PMC article. Review. - Crowding-induced phase separation of nuclear transport receptors in FG nucleoporin assemblies.
Davis LK, Ford IJ, Hoogenboom BW. Davis LK, et al. Elife. 2022 Jan 31;11:e72627. doi: 10.7554/eLife.72627. Elife. 2022. PMID: 35098921 Free PMC article. - Biomechanics of the transport barrier in the nuclear pore complex.
Stanley GJ, Fassati A, Hoogenboom BW. Stanley GJ, et al. Semin Cell Dev Biol. 2017 Aug;68:42-51. doi: 10.1016/j.semcdb.2017.05.007. Epub 2017 May 12. Semin Cell Dev Biol. 2017. PMID: 28506890 Review. - The mechanism of nucleocytoplasmic transport through the nuclear pore complex.
Tetenbaum-Novatt J, Rout MP. Tetenbaum-Novatt J, et al. Cold Spring Harb Symp Quant Biol. 2010;75:567-84. doi: 10.1101/sqb.2010.75.033. Epub 2011 Mar 29. Cold Spring Harb Symp Quant Biol. 2010. PMID: 21447814 - Super-resolution mapping of scaffold nucleoporins in the nuclear pore complex.
Ma J, Kelich JM, Junod SL, Yang W. Ma J, et al. J Cell Sci. 2017 Apr 1;130(7):1299-1306. doi: 10.1242/jcs.193912. Epub 2017 Feb 15. J Cell Sci. 2017. PMID: 28202688 Free PMC article.
Cited by
- Nucleocytoplasmic transport of intrinsically disordered proteins studied by high-speed super-resolution microscopy.
Junod SL, Kelich JM, Ma J, Yang W. Junod SL, et al. Protein Sci. 2020 Jun;29(6):1459-1472. doi: 10.1002/pro.3845. Epub 2020 Mar 3. Protein Sci. 2020. PMID: 32096308 Free PMC article. - Nuclear Transport and Accumulation of Smad Proteins Studied by Single-Molecule Microscopy.
Li Y, Luo W, Yang W. Li Y, et al. Biophys J. 2018 May 8;114(9):2243-2251. doi: 10.1016/j.bpj.2018.03.018. Biophys J. 2018. PMID: 29742417 Free PMC article. - Quantitative comparison of nuclear transport inhibition by SARS coronavirus ORF6 reveals the importance of oligomerization.
Yoo TY, Mitchison TJ. Yoo TY, et al. Proc Natl Acad Sci U S A. 2024 Jan 23;121(4):e2307997121. doi: 10.1073/pnas.2307997121. Epub 2024 Jan 18. Proc Natl Acad Sci U S A. 2024. PMID: 38236733 Free PMC article. - Self-regulation of the nuclear pore complex enables clogging-free crowded transport.
Zheng T, Zilman A. Zheng T, et al. Proc Natl Acad Sci U S A. 2023 Feb 14;120(7):e2212874120. doi: 10.1073/pnas.2212874120. Epub 2023 Feb 9. Proc Natl Acad Sci U S A. 2023. PMID: 36757893 Free PMC article. - Multiple types of nuclear localization signals in Entamoeba histolytica.
Canela-Pérez I, Azuara-Liceaga E, Cuéllar P, Saucedo-Cárdenas O, Valdés J. Canela-Pérez I, et al. Biochem Biophys Rep. 2024 Jul 4;39:101770. doi: 10.1016/j.bbrep.2024.101770. eCollection 2024 Sep. Biochem Biophys Rep. 2024. PMID: 39055170 Free PMC article.
References
- Suntharalingam M, Wente SR. Peering through the pore: nuclear pore complex structure, assembly, and function. Dev Cell. 2003;4:775–789. - PubMed
- Fahrenkrog B, Aebi U. The nuclear pore complex: nucleocytoplasmic transport and beyond. Nat Rev Mol Cell Biol. 2003;4:757–766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM116204/GM/NIGMS NIH HHS/United States
- GM094041/GM/NIGMS NIH HHS/United States
- GM097037/GM/NIGMS NIH HHS/United States
- R15 GM094041/GM/NIGMS NIH HHS/United States
- R01 GM097037/GM/NIGMS NIH HHS/United States
- R01 GM116204/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources