The role of CHMP2BIntron5 in autophagy and frontotemporal dementia - PubMed (original) (raw)
Review
The role of CHMP2BIntron5 in autophagy and frontotemporal dementia
Christopher S Krasniak et al. Brain Res. 2016.
Abstract
Charged multivesicular body protein 2B (CHMP2B) - a component of the endosomal complex required for transport-III (ESCRT-III) - is responsible for the vital membrane deformation functions in autophagy and endolysosomal trafficking. A dominant mutation in CHMP2B (CHMP2BIntron5) is associated with a subset of heritable frontotemporal dementia - frontotemporal dementia linked to chromosome 3 (FTD-3). ESCRT-III recruits Vps4, an AAA-ATPase that abscises the membrane during various cellular processes including autophagy and intraluminal vesicle formation. CHMP2BIntron5 results in a C-terminus truncation removing an important Vps4 binding site as well as eliminating the normal autoinhibitory resting state of CHMP2B. CHMP2B is expressed in most cell types but seems to be especially vital for proper neuronal function. CHMP2BIntron5-mediated phenotypes include misregulation of transmembrane receptors, accumulation of multilamellar structures, abnormal lysosomal morphology, down regulation of a brain-specific micro RNA (miRNA-124), abnormal dendritic spine morphology, decrease in dendritic arborization, and cell death. Currently, transgenic-fly,-mouse, and -human cell lines are being used to better understand the diverse phenotypes and develop therapeutic approaches for the CHMP2BIntron5-induced FTD-3. This article is part of a Special Issue entitled SI:Autophagy.
Keywords: Autophagy; CHMP2B(Intron5); ESCRT-III; Endolysosome; FTD-ALS; Frontotemporal dementia.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures
Figure 1
Mutations in the CHMP2B gene causing FTD and other neurodegenerative diseases. A. There are several CHMP2B mutations that have been found to cause diseases on the FTD-ALS spectrum (I29V, T104N, D148Y, Q165X, M178V, and Q206H; orange boxes) as well as other neurodegenerative disorders; corticobasal degeneration caused by N143S (purple box). B. Functional wild type CHMP2B domains and altered CHMP2BIntron5 protein. Wild type CHMP2B is a 213 amino acid protein with two coiled-coil (CC) domains as well as a microtubule interacting and transport interacting motif (MIM). The N-terminus contains basic alpha helices and the C-terminus contains acidic alpha helices, leading to an auto-inhibitory self-binding. CHMP2BIntron5 loses much of the acidic C-terminus, decreasing its autoinhibition and removing the MIM, which is a Vps4 binding site. E, Exon; PMA, primary muscular atrophy; CBD, corticobasal degeneration; SD, semantic dementia; FTD, frontotemporal dementia; * denotes mutation causing CHMP2BIntron5 and CHMP2BDelta10.
Figure 2
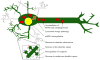
CHMP2BIntron5 causes a diverse array of neuronal pathologies. The known molecular and structural bases of this neurodegeneration include: misregulation of miRNA, accumulation of autophagosomes and MVBs, abnormally sized lysosomes, a decrease in dendritic arborization, an overall increase in dendritic spines but with a decrease in mushroom spines, and misregulation of receptors such as Notch, and EGFR. These pathologies combined not only weaken the cell, but also leads to death of many frontal and temporal neurons.
Similar articles
- TMEM106B, a frontotemporal lobar dementia (FTLD) modifier, associates with FTD-3-linked CHMP2B, a complex of ESCRT-III.
Jun MH, Han JH, Lee YK, Jang DJ, Kaang BK, Lee JA. Jun MH, et al. Mol Brain. 2015 Dec 10;8:85. doi: 10.1186/s13041-015-0177-z. Mol Brain. 2015. PMID: 26651479 Free PMC article. - Expression of mutant CHMP2B, an ESCRT-III component involved in frontotemporal dementia, causes eye deformities due to Notch misregulation in Drosophila.
Cheruiyot A, Lee JA, Gao FB, Ahmad ST. Cheruiyot A, et al. FASEB J. 2014 Feb;28(2):667-75. doi: 10.1096/fj.13-234138. Epub 2013 Oct 24. FASEB J. 2014. PMID: 24158394 Free PMC article. - A transgenic mouse expressing CHMP2Bintron5 mutant in neurons develops histological and behavioural features of amyotrophic lateral sclerosis and frontotemporal dementia.
Vernay A, Therreau L, Blot B, Risson V, Dirrig-Grosch S, Waegaert R, Lequeu T, Sellal F, Schaeffer L, Sadoul R, Loeffler JP, René F. Vernay A, et al. Hum Mol Genet. 2016 Aug 1;25(15):3341-3360. doi: 10.1093/hmg/ddw182. Epub 2016 Jun 21. Hum Mol Genet. 2016. PMID: 27329763 - Molecular Genetics of Frontotemporal Dementia Elucidated by Drosophila Models-Defects in Endosomal⁻Lysosomal Pathway.
Vandal SE, Zheng X, Ahmad ST. Vandal SE, et al. Int J Mol Sci. 2018 Jun 9;19(6):1714. doi: 10.3390/ijms19061714. Int J Mol Sci. 2018. PMID: 29890743 Free PMC article. Review. - Frontotemporal dementia caused by CHMP2B mutations.
Isaacs AM, Johannsen P, Holm I, Nielsen JE; FReJA consortium. Isaacs AM, et al. Curr Alzheimer Res. 2011 May;8(3):246-51. doi: 10.2174/156720511795563764. Curr Alzheimer Res. 2011. PMID: 21222599 Free PMC article. Review.
Cited by
- Astrocytic reactivity triggered by defective autophagy and metabolic failure causes neurotoxicity in frontotemporal dementia type 3.
Chandrasekaran A, Dittlau KS, Corsi GI, Haukedal H, Doncheva NT, Ramakrishna S, Ambardar S, Salcedo C, Schmidt SI, Zhang Y, Cirera S, Pihl M, Schmid B, Nielsen TT, Nielsen JE, Kolko M, Kobolák J, Dinnyés A, Hyttel P, Palakodeti D, Gorodkin J, Muddashetty RS, Meyer M, Aldana BI, Freude KK. Chandrasekaran A, et al. Stem Cell Reports. 2021 Nov 9;16(11):2736-2751. doi: 10.1016/j.stemcr.2021.09.013. Epub 2021 Oct 21. Stem Cell Reports. 2021. PMID: 34678206 Free PMC article. - Exploring Intrinsic Disorder in Human Synucleins and Associated Proteins.
Venati SR, Uversky VN. Venati SR, et al. Int J Mol Sci. 2024 Aug 1;25(15):8399. doi: 10.3390/ijms25158399. Int J Mol Sci. 2024. PMID: 39125972 Free PMC article. - Autophagy and neurodegeneration: Unraveling the role of C9ORF72 in the regulation of autophagy and its relationship to ALS-FTD pathology.
Diab R, Pilotto F, Saxena S. Diab R, et al. Front Cell Neurosci. 2023 Mar 16;17:1086895. doi: 10.3389/fncel.2023.1086895. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37006471 Free PMC article. Review. - Factor Analysis for Bicluster Acquisition (FABIA) revealed vincristine-sensitive transcript pattern of canine transmissible venereal tumors.
Chokeshaiusaha K, Puthier D, Nguyen C, Sudjaidee P, Sananmuang T. Chokeshaiusaha K, et al. Heliyon. 2019 May 14;5(5):e01558. doi: 10.1016/j.heliyon.2019.e01558. eCollection 2019 May. Heliyon. 2019. PMID: 31193204 Free PMC article. - The ESCRT-Related ATPase Vps4 Is Modulated by Interferon during Herpes Simplex Virus 1 Infection.
Cabrera JR, Manivanh R, North BJ, Leib DA. Cabrera JR, et al. mBio. 2019 Mar 5;10(2):e02567-18. doi: 10.1128/mBio.02567-18. mBio. 2019. PMID: 30837340 Free PMC article.
References
- Ahmad ST, Lee J-A. Molecular Mechanisms Underlying the Role of Autophagy in Neurodegenerative Diseases. 2014:45–59.
- Anderson KN, et al. Disrupted sleep and circadian patterns in frontotemporal dementia. Eur J Neurol. 2009;16:317–23. - PubMed
- Baker M, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous