Mass spectrometric analysis of accumulated TDP-43 in amyotrophic lateral sclerosis brains - PubMed (original) (raw)
Mass spectrometric analysis of accumulated TDP-43 in amyotrophic lateral sclerosis brains
Fuyuki Kametani et al. Sci Rep. 2016.
Abstract
TDP-43 is the major disease-associated protein involved in the pathogenesis and progression of amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration with ubiquitin-positive inclusions linked to TDP-43 pathology (FTLD-TDP). Abnormal phosphorylation, truncation and cytoplasmic mis-localization are known to be the characteristics for the aggregated forms of TDP-43, and gain of toxic abnormal TDP-43 or loss of function of physiological TDP-43 have been suggested as the cause of neurodegeneration. However, most of the post-translational modifications or truncation sites in the abnormal TDP-43 in brains of patients remain to be identified by protein chemical analysis. In this study, we carried out a highly sensitive liquid chromatography-mass spectrometry analysis of Sarkosyl-insoluble pathological TDP-43 from brains of ALS patients and identified several novel phosphorylation sites, deamidation sites, and cleavage sites. Almost all modifications were localized in the Gly-rich C-terminal half. Most of the cleavage sites identified in this study are novel and are located in N-terminal half, suggesting that these sites may be more accessible to proteolytic enzymes. The data obtained in this study provide a foundation for the molecular mechanisms of TDP-43 aggregation and ALS pathogenesis.
Figures
Figure 1. SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotting of Sarkosyl-insoluble fraction from ALS case 1 brain and ALS case 2 brain.
(A) A phosphorylation-independent anti-TDP-43 detected normal TDP-43 of 43 kD in the fractions of both ALS and control brains, but also detected abnormal TDP-43 bands, including full-length phosphorylated TDP-43 of 45 kD, ~25 kD fragments and smears in ALS brains. (B) TDP-43-positive bands were excised as indicated.
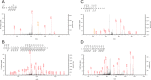
Figure 2. MS/MS identification of phosphorylated peptides and cleavage site peptides.
Representative peptides were shown. (A) Chymotriptic peptide, 398-NGGFGSSM-405 in case 1. The 6th Ser residue was phosphorylated. (B)Triptic peptide, 56-LVEGILHAPDAGWGNLVYVVNYPK-79 in case 1. (C) Tryptic peptide, 215- GDVMDVFIPKPFR-227 in case 1. Trypsin can not cleave N-terminal Tyr214-Gly215 site. Therefore this site is intrinsically cleaved site. (D) Chymotryptic peptide, 56-LVEGILHAPDAGW-68 in case 2. Chymotrypsin can not cleave N-terminal Arg55-Leu56 site. Therefore this site is intrinsically cleaved site.
Figure 3
Identification of modification sites in TDP-43 from ALS case 1 (A) and case 2 (B) by LC-MS/MS analysis. Identified peptides from trypsin digestion (light green) and from chymotrypsin digestion (dark green) are shown. p indicates phosphorylation site. o indicates oxidation site. * indicates deamidation site. Blue and pink arrows indicate N-terminal and C-terminal cleavage sites, respectively.
Figure 4. Schematic representation of N-terminal and C-terminal cleavage sites on TDP-43.
Cleavage sites (amino acid numbering of TDP-43) in gel fractions and antibodies recognition sites were shown.
Similar articles
- Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis.
Hasegawa M, Arai T, Nonaka T, Kametani F, Yoshida M, Hashizume Y, Beach TG, Buratti E, Baralle F, Morita M, Nakano I, Oda T, Tsuchiya K, Akiyama H. Hasegawa M, et al. Ann Neurol. 2008 Jul;64(1):60-70. doi: 10.1002/ana.21425. Ann Neurol. 2008. PMID: 18546284 Free PMC article. - TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis.
Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. Arai T, et al. Biochem Biophys Res Commun. 2006 Dec 22;351(3):602-11. doi: 10.1016/j.bbrc.2006.10.093. Epub 2006 Oct 30. Biochem Biophys Res Commun. 2006. PMID: 17084815 - Abnormal phosphorylation of Ser409/410 of TDP-43 in FTLD-U and ALS.
Inukai Y, Nonaka T, Arai T, Yoshida M, Hashizume Y, Beach TG, Buratti E, Baralle FE, Akiyama H, Hisanaga S, Hasegawa M. Inukai Y, et al. FEBS Lett. 2008 Aug 20;582(19):2899-904. doi: 10.1016/j.febslet.2008.07.027. Epub 2008 Jul 24. FEBS Lett. 2008. PMID: 18656473 - Phosphorylated and cleaved TDP-43 in ALS, FTLD and other neurodegenerative disorders and in cellular models of TDP-43 proteinopathy.
Arai T, Hasegawa M, Nonoka T, Kametani F, Yamashita M, Hosokawa M, Niizato K, Tsuchiya K, Kobayashi Z, Ikeda K, Yoshida M, Onaya M, Fujishiro H, Akiyama H. Arai T, et al. Neuropathology. 2010 Apr;30(2):170-81. doi: 10.1111/j.1440-1789.2009.01089.x. Epub 2010 Jan 19. Neuropathology. 2010. PMID: 20102522 Review. - Possible concurrence of TDP-43, tau and other proteins in amyotrophic lateral sclerosis/frontotemporal lobar degeneration.
Takeda T. Takeda T. Neuropathology. 2018 Feb;38(1):72-81. doi: 10.1111/neup.12428. Epub 2017 Sep 27. Neuropathology. 2018. PMID: 28960544 Review.
Cited by
- Importin α/β and the tug of war to keep TDP-43 in solution: quo vadis?
Doll SG, Cingolani G. Doll SG, et al. Bioessays. 2022 Dec;44(12):e2200181. doi: 10.1002/bies.202200181. Epub 2022 Oct 17. Bioessays. 2022. PMID: 36253101 Free PMC article. Review. - Aggregation of the nucleic acid-binding protein TDP-43 occurs via distinct routes that are coordinated with stress granule formation.
Chen Y, Cohen TJ. Chen Y, et al. J Biol Chem. 2019 Mar 8;294(10):3696-3706. doi: 10.1074/jbc.RA118.006351. Epub 2019 Jan 10. J Biol Chem. 2019. PMID: 30630951 Free PMC article. - Metamorphism in TDP-43 prion-like domain determines chaperone recognition.
Carrasco J, Antón R, Valbuena A, Pantoja-Uceda D, Mukhi M, Hervás R, Laurents DV, Gasset M, Oroz J. Carrasco J, et al. Nat Commun. 2023 Jan 28;14(1):466. doi: 10.1038/s41467-023-36023-z. Nat Commun. 2023. PMID: 36709343 Free PMC article. - Frontotemporal lobar degeneration with TAR DNA-binding protein 43 (TDP-43): its journey of more than 100 years.
Carlos AF, Josephs KA. Carlos AF, et al. J Neurol. 2022 Aug;269(8):4030-4054. doi: 10.1007/s00415-022-11073-3. Epub 2022 Mar 23. J Neurol. 2022. PMID: 35320398 Free PMC article. Review. - The Pathobiology of TDP-43 C-Terminal Fragments in ALS and FTLD.
Berning BA, Walker AK. Berning BA, et al. Front Neurosci. 2019 Apr 11;13:335. doi: 10.3389/fnins.2019.00335. eCollection 2019. Front Neurosci. 2019. PMID: 31031584 Free PMC article. Review.
References
- Corrado L. et al. High frequency of TARDBP gene mutations in Italian patients with amyotrophic lateral sclerosis. Human Mutation 30, 688–694 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous