Driving CAR T-cells forward - PubMed (original) (raw)
Review
Driving CAR T-cells forward
Hollie J Jackson et al. Nat Rev Clin Oncol. 2016 Jun.
Abstract
The engineered expression of chimeric antigen receptors (CARs) on the surface of T cells enables the redirection of T-cell specificity. Early clinical trials using CAR T cells for the treatment of patients with cancer showed modest results, but the impressive outcomes of several trials of CD19-targeted CAR T cells in the treatment of patients with B-cell malignancies have generated an increased enthusiasm for this approach. Important lessons have been derived from clinical trials of CD19-specific CAR T cells, and ongoing clinical trials are testing CAR designs directed at novel targets involved in haematological and solid malignancies. In this Review, we discuss these trials and present strategies that can increase the antitumour efficacy and safety of CAR T-cell therapy. Given the fast-moving nature of this field, we only discuss studies with direct translational application currently or soon-to-be tested in the clinical setting.
Conflict of interest statement
Competing interests statement
H.J.J. and S.R. declare no competing interests. R.J.B. is a co-founder, stockholder and consultant for Juno Therapeutics Inc.
Figures
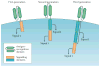
Figure 1. CAR-T-cell design
All chimeric antigen receptor (CAR) designs contain an antigen-recognition domain and a signalling domain that provides ‘signal 1’ to activate T cells. Only this signalling domain is present in first-generation CARs. By contrast, a co-stimulatory signalling domain that provides ‘signal 2’ is added in second-generation CARs, and in third-generation CARs two co-stimulatory signalling domains are added.
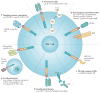
Figure 2. Approaches to improve CAR-T-cell therapy
An overview of the improvements to chimeric antigen receptor (CAR)-T-cell therapy is shown, and clinical trials testing those strategies are indicated in the legend. a | Engineered CAR T cells that secrete pro-inflammatory cytokines (armoured CAR T cells; NCT02498912). b | Dual receptor expression to target tumour cells and convert tumour-derived cytokines into T-cell activators (NCT01818323). c | Using natural killer (NK)-cell-based recognition domains, such as NKG2-D, in CARs (NCT02203825). d | Combination therapy with monoclonal antibodies (mAb) targeting immune-checkpoint inhibitory receptors to relieve immunosuppression (NCT00586391). e | Infusion of two populations of CAR T cells to eradicate B cells and enable increased persistence of tumour-specific CAR T cells by preventing antibody responses against their foreign antigen components (NCT02465983). f | Targeting the tumour vasculature with CAR T cells, such as VEGFR-2-specific CAR T cells NCT01218867. 4αβ, 4αβ chimeric cytokine receptor; CTLA-4, cytotoxic T-lymphocyte-associate antigen-4; T1E28z, T1E28z chimeric antigen receptor; VEGFR-2, vascular endothelial growth factor receptor 2.
Similar articles
- Treatment of patients with relapsed or refractory CD19+ lymphoid disease with T lymphocytes transduced by RV-SFG.CD19.CD28.4-1BBzeta retroviral vector: a unicentre phase I/II clinical trial protocol.
Schubert ML, Schmitt A, Sellner L, Neuber B, Kunz J, Wuchter P, Kunz A, Gern U, Michels B, Hofmann S, Hückelhoven-Krauss A, Kulozik A, Ho AD, Müller-Tidow C, Dreger P, Schmitt M. Schubert ML, et al. BMJ Open. 2019 May 19;9(5):e026644. doi: 10.1136/bmjopen-2018-026644. BMJ Open. 2019. PMID: 31110096 Free PMC article. Clinical Trial. - Making Better Chimeric Antigen Receptors for Adoptive T-cell Therapy.
Maus MV, June CH. Maus MV, et al. Clin Cancer Res. 2016 Apr 15;22(8):1875-84. doi: 10.1158/1078-0432.CCR-15-1433. Clin Cancer Res. 2016. PMID: 27084741 Free PMC article. Review. - A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines.
Schneider D, Xiong Y, Wu D, Nӧlle V, Schmitz S, Haso W, Kaiser A, Dropulic B, Orentas RJ. Schneider D, et al. J Immunother Cancer. 2017 May 16;5:42. doi: 10.1186/s40425-017-0246-1. eCollection 2017. J Immunother Cancer. 2017. PMID: 28515942 Free PMC article. - Antitumor Potency of an Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy, Lisocabtagene Maraleucel in Combination With Ibrutinib or Acalabrutinib.
Qin JS, Johnstone TG, Baturevych A, Hause RJ, Ragan SP, Clouser CR, Jones JC, Ponce R, Krejsa CM, Salmon RA, Ports MO. Qin JS, et al. J Immunother. 2020 May;43(4):107-120. doi: 10.1097/CJI.0000000000000307. J Immunother. 2020. PMID: 31899702 Free PMC article. - Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: lessons learned and strategies for moving forward.
Li J, Li W, Huang K, Zhang Y, Kupfer G, Zhao Q. Li J, et al. J Hematol Oncol. 2018 Feb 13;11(1):22. doi: 10.1186/s13045-018-0568-6. J Hematol Oncol. 2018. PMID: 29433552 Free PMC article. Review.
Cited by
- CAR-T cell therapy: Advances in digestive system malignant tumors.
Xu N, Wu Z, Pan J, Xu X, Wei Q. Xu N, et al. Mol Ther Oncol. 2024 Sep 10;32(4):200872. doi: 10.1016/j.omton.2024.200872. eCollection 2024 Dec 19. Mol Ther Oncol. 2024. PMID: 39377038 Free PMC article. Review. - Effect of cabazitaxel on macrophages improves CD47-targeted immunotherapy for triple-negative breast cancer.
Cao X, Li B, Chen J, Dang J, Chen S, Gunes EG, Xu B, Tian L, Muend S, Raoof M, Querfeld C, Yu J, Rosen ST, Wang Y, Feng M. Cao X, et al. J Immunother Cancer. 2021 Mar;9(3):e002022. doi: 10.1136/jitc-2020-002022. J Immunother Cancer. 2021. PMID: 33753567 Free PMC article. - Killers 2.0: NK cell therapies at the forefront of cancer control.
Hodgins JJ, Khan ST, Park MM, Auer RC, Ardolino M. Hodgins JJ, et al. J Clin Invest. 2019 Sep 3;129(9):3499-3510. doi: 10.1172/JCI129338. J Clin Invest. 2019. PMID: 31478911 Free PMC article. Review. - MRD-Negative Remission Induced in EP300-ZNF384 Positive B-ALL Patients by Tandem CD19/CD22 CAR T-Cell Therapy Bridging to Allogeneic Stem Cell Transplantation.
Zhang XY, Dai HP, Zhang L, Liu SN, Dai Y, Wu DP, Tang XW. Zhang XY, et al. Onco Targets Ther. 2021 Oct 29;14:5197-5204. doi: 10.2147/OTT.S324765. eCollection 2021. Onco Targets Ther. 2021. PMID: 34744437 Free PMC article. - CRISPR/Cas9: A Powerful Strategy to Improve CAR-T Cell Persistence.
Wei W, Chen ZN, Wang K. Wei W, et al. Int J Mol Sci. 2023 Aug 1;24(15):12317. doi: 10.3390/ijms241512317. Int J Mol Sci. 2023. PMID: 37569693 Free PMC article. Review.
References
- Lamers CH, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–e22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources