Theaflavin-3, 3'-digallate induces apoptosis and G2 cell cycle arrest through the Akt/MDM2/p53 pathway in cisplatin-resistant ovarian cancer A2780/CP70 cells - PubMed (original) (raw)
Theaflavin-3, 3'-digallate induces apoptosis and G2 cell cycle arrest through the Akt/MDM2/p53 pathway in cisplatin-resistant ovarian cancer A2780/CP70 cells
Youying Tu et al. Int J Oncol. 2016 Jun.
Abstract
Ovarian cancer is the most lethal gynecological cancer among women worldwide. Adverse side effects and acquired resistance to conventional platinum based chemotherapy are major impediments in ovarian cancer treatment, and drive the development of more selective anticancer drugs that target cancer-specific defects. In this study, theaflavin-3, 3'-digallate (TF3), the major theaflavin monomer in black tea, exhibited a potent growth inhibitory effect on the cisplatin-resistant ovarian cancer A2780/CP70 cells (IC50, 23.81 µM), and was less cytotoxic to a normal ovarian IOSE‑364 cells (IC50, 59.58 µM) than to the cancer cells. Flow cytometry analysis indicated that TF3 induced preferential apoptosis and G2 cell cycle arrest in A2780/CP70 cells with respect to IOSE‑364 cells. TF3 induced apoptosis through both the intrinsic and extrinsic apoptotic pathways, and caused G2 cell cycle arrest via cyclin B1 in A2780/CP70 cells. The p53 protein played an important role in TF3-induced apoptosis and G2 cell cycle arrest. TF3 might upregulate the p53 expression via the Akt/MDM2 pathway. Our findings help elucidate the mechanisms by which TF3 may contribute to the prevention and treatment of platinum-resistant ovarian cancer.
Figures
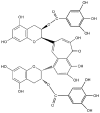
Figure 1
Chemical structure of TF3 used in this study.
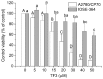
Figure 2
Effect of TF3 on cell growth in human ovarian cancer cells A2780/ CP70 and normal ovarian cells IOSE-364. Cells (1×104/well) were seeded in 96-well plates, incubated overnight, and then treated with TF3 for 24 h. Cell viability was determined by MTT assay. Data represent means ± SD from three independent experiments. Significant differences among the treatments are indicated by different letters (p<0.05).
Figure 3
TF3 induces apoptosis in A2780/CP70 and IOSE-364 cells. (A) Flow cytometry of TF3 treated A2780/CP70 and IOSE-364 cells using a double-staining method with FITC-conjugated Annexin V and PI. The upper left and low left quadrant indicate the percentage of dead and live cells, respectively, and the upper right and low right quadrants indicate the percentage of late and early apoptotic cells, respectively. (B) Caspase-3/7 activity levels with TF3-treatment for 24 h. The caspase-3/7 activity of the control cells after treatment was arbitrarily expressed as 100%. Data represent means ± SD of three independent experiments. Significant differences among the treatments are indicated by different letters (p<0.05).
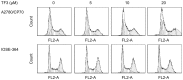
Figure 4
TF3 induces cell cycle arrest in A2780/CP70 and IOSE-364 cells. Cells were treated with various concentrations (0, 5, 10 and 20 μM) of TF3 for 24 h, fixed in 70% ethanol, and stained with propidium iodide. DNA contents were determined by flow cytometry.
Figure 5
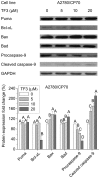
Effect of TF3 on the intrinsic apoptotic pathway in A2780/CP70 cells. Protein lysates were prepared from A2780/CP70 cells after treatment with various concentrations (0, 5, 10 and 20 μM) of TF3 for 24 h. Puma, Bax, Bad, Bcl-xL and caspase-9 protein levels were analyzed by western blotting. The quantification histograms are shown with error bars. Data represent means ± SD from three independent experiments. Significant differences among the treatments are indicated by different letters (p<0.05).
Figure 6
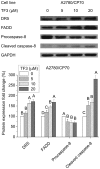
Effect of TF3 on the extrinsic apoptotic pathway in A2780/CP70 cells. Protein lysates were prepared from A2780/CP70 cells after treatment with various concentrations (0, 5, 10 and 20 μM) of TF3 for 24 h. DR5, FADD, procaspase-8 and cleaved caspase-8 protein levels were analyzed by western blotting. The quantification histograms are shown with error bars. Data represent means ± SD from three independent experiments. Significant differences among different treatments are indicated by different letters (p<0.05).
Figure 7
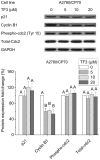
Effect of TF3 on cell cycle G2-related proteins in A2780/CP70 cells. Protein lysates were prepared from A2780/CP70 cells after treatment with various concentrations (0, 5, 10 and 20 μM) of TF3 for 24 h. p21, cyclin B1, phospho-cdc2 and total-cdc2 protein expression levels were analyzed by western blotting. The quantification histograms are shown with error bars. Data represent means ± SD from three independent experiments. Significant differences among the treatments are indicated by different letters (p<0.05).
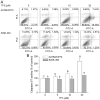
Figure 8
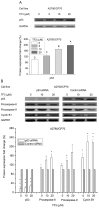
Role of p53 in TF3-induced apoptosis and G2 cell cycle arrest of A2780/CP70 cells. The quantification histograms are shown with error bars. Data represent means ± SD from three independent experiments. (A) The effects of TF3 on the protein expression of p53 determined by western blotting. Significant differences among the treatments are indicated by different letters (p<0.05). (B) The effects of p53 siRNA (50 nM) on the protein expression of procaspase-8, procaspase-9 and cyclin B1 determined by western blotting. *p<0.05, **p<0.01, compared with respective controls.
Figure 9
Effect of TF3 on Akt/MDM2 pathway in A2780/CP70 cells. Protein lysates were prepared from A2780/CP70 cells after treatment with various concentrations (0, 5, 10 and 20 μM) of TF3 for 24 h. phospho-Akt, total-Akt and MDM2 protein expression levels were analyzed by western blotting. The quantification histograms are shown with error bars. Data represent means ± SD from three independent experiments. Significant differences among the treatments are indicated by different letters (p<0.05).
Similar articles
- Inhibitory effect of black tea pigments, theaflavin‑3/3'-gallate against cisplatin-resistant ovarian cancer cells by inducing apoptosis and G1 cell cycle arrest.
Pan H, Wang F, Rankin GO, Rojanasakul Y, Tu Y, Chen YC. Pan H, et al. Int J Oncol. 2017 Nov;51(5):1508-1520. doi: 10.3892/ijo.2017.4145. Epub 2017 Oct 3. Int J Oncol. 2017. PMID: 29048667 Free PMC article. - Chaetoglobosin K induces apoptosis and G2 cell cycle arrest through p53-dependent pathway in cisplatin-resistant ovarian cancer cells.
Li B, Gao Y, Rankin GO, Rojanasakul Y, Cutler SJ, Tu Y, Chen YC. Li B, et al. Cancer Lett. 2015 Jan 28;356(2 Pt B):418-33. doi: 10.1016/j.canlet.2014.09.023. Epub 2014 Oct 7. Cancer Lett. 2015. PMID: 25304379 Free PMC article. - Theaflavin-3,3'-Digallate Suppresses Human Ovarian Carcinoma OVCAR-3 Cells by Regulating the Checkpoint Kinase 2 and p27 kip1 Pathways.
Gao Y, Yin J, Tu Y, Chen YC. Gao Y, et al. Molecules. 2019 Feb 14;24(4):673. doi: 10.3390/molecules24040673. Molecules. 2019. PMID: 30769778 Free PMC article. - Molecular determinants of ovarian cancer chemoresistance: new insights into an old conundrum.
Ali AY, Farrand L, Kim JY, Byun S, Suh JY, Lee HJ, Tsang BK. Ali AY, et al. Ann N Y Acad Sci. 2012 Oct;1271(1):58-67. doi: 10.1111/j.1749-6632.2012.06734.x. Ann N Y Acad Sci. 2012. PMID: 23050965 Free PMC article. Review. - Role of FOXO protein's abnormal activation through PI3K/AKT pathway in platinum resistance of ovarian cancer.
Shi YY, Meng XT, Xu YN, Tian XJ. Shi YY, et al. J Obstet Gynaecol Res. 2021 Jun;47(6):1946-1957. doi: 10.1111/jog.14753. Epub 2021 Apr 7. J Obstet Gynaecol Res. 2021. PMID: 33827148 Review.
Cited by
- Purified Tea (Camellia sinensis (L.) Kuntze) Flower Saponins Induce the p53-Dependent Intrinsic Apoptosis of Cisplatin-Resistant Ovarian Cancer Cells.
Ren N, Chen L, Li B, Rankin GO, Chen YC, Tu Y. Ren N, et al. Int J Mol Sci. 2020 Jun 17;21(12):4324. doi: 10.3390/ijms21124324. Int J Mol Sci. 2020. PMID: 32560563 Free PMC article. - Theaflavins Improve Insulin Sensitivity through Regulating Mitochondrial Biosynthesis in Palmitic Acid-Induced HepG2 Cells.
Tong T, Ren N, Soomi P, Wu J, Guo N, Kang H, Kim E, Wu Y, He P, Tu Y, Li B. Tong T, et al. Molecules. 2018 Dec 19;23(12):3382. doi: 10.3390/molecules23123382. Molecules. 2018. PMID: 30572687 Free PMC article. - ROS play an important role in ATPR inducing differentiation and inhibiting proliferation of leukemia cells by regulating the PTEN/PI3K/AKT signaling pathway.
Feng Y, Hua X, Niu R, Du Y, Shi C, Zhou R, Chen FH. Feng Y, et al. Biol Res. 2019 May 3;52(1):26. doi: 10.1186/s40659-019-0232-9. Biol Res. 2019. PMID: 31053167 Free PMC article. - The role of CDC25C in cell cycle regulation and clinical cancer therapy: a systematic review.
Liu K, Zheng M, Lu R, Du J, Zhao Q, Li Z, Li Y, Zhang S. Liu K, et al. Cancer Cell Int. 2020 Jun 3;20:213. doi: 10.1186/s12935-020-01304-w. eCollection 2020. Cancer Cell Int. 2020. PMID: 32518522 Free PMC article. Review. - Standardized Saponin Extract from Baiye No.1 Tea (Camellia sinensis) Flowers Induced S Phase Cell Cycle Arrest and Apoptosis via AKT-MDM2-p53 Signaling Pathway in Ovarian Cancer Cells.
Tu Y, Chen L, Ren N, Li B, Wu Y, Rankin GO, Rojanasakul Y, Wang Y, Chen YC. Tu Y, et al. Molecules. 2020 Jul 31;25(15):3515. doi: 10.3390/molecules25153515. Molecules. 2020. PMID: 32752095 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous