Endotoxin Disrupts Circadian Rhythms in Macrophages via Reactive Oxygen Species - PubMed (original) (raw)
Endotoxin Disrupts Circadian Rhythms in Macrophages via Reactive Oxygen Species
Yusi Wang et al. PLoS One. 2016.
Abstract
The circadian clock is a transcriptional network that functions to regulate the expression of genes important in the anticipation of changes in cellular and organ function. Recent studies have revealed that the recognition of pathogens and subsequent initiation of inflammatory responses are strongly regulated by a macrophage-intrinsic circadian clock. We hypothesized that the circadian pattern of gene expression might be influenced by inflammatory stimuli and that loss of circadian function in immune cells can promote pro-inflammatory behavior. To investigate circadian rhythms in inflammatory cells, peritoneal macrophages were isolated from mPer2luciferase transgenic mice and circadian oscillations were studied in response to stimuli. Using Cosinor analysis, we found that LPS significantly altered the circadian period in peritoneal macrophages from mPer2luciferase mice while qPCR data suggested that the pattern of expression of the core circadian gene (Bmal1) was disrupted. Inhibition of TLR4 offered protection from the LPS-induced impairment in rhythm, suggesting a role for toll-like receptor signaling. To explore the mechanisms involved, we inhibited LPS-stimulated NO and superoxide. Inhibition of NO synthesis with L-NAME had no effect on circadian rhythms. In contrast, inhibition of superoxide with Tempol or PEG-SOD ameliorated the LPS-induced changes in circadian periodicity. In gain of function experiments, we found that overexpression of NOX5, a source of ROS, could significantly disrupt circadian function in a circadian reporter cell line (U2OS) whereas iNOS overexpression, a source of NO, was ineffective. To assess whether alteration of circadian rhythms influences macrophage function, peritoneal macrophages were isolated from Bmal1-KO and Per-TKO mice. Compared to WT macrophages, macrophages from circadian knockout mice exhibited altered balance between NO and ROS release, increased uptake of oxLDL and increased adhesion and migration. These results suggest that pro-inflammatory stimuli can disrupt circadian rhythms in macrophages and that impaired circadian rhythms may contribute to cardiovascular diseases by altering macrophage behavior.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
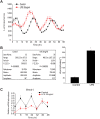
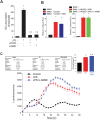
Fig 1. LPS induces a phase shift in synchronized peritoneal macrophages and impairs the expression of core circadian genes in peritoneal macrophages.
(A) Peritoneal macrophages from _mPer2_luciferase transgenic mice were seeded in 96-well plate (white) for 24 hours. After 2h serum shock, cells were kept in luminescence buffer in presence or absence of LPS (20ng/ml) and bioluminescence was recorded every 2 hours which starts from Time 0. (B) Oscillation curves were analyzed by cosinor (time 0 and 24h represented by 0° and 360°, respectfully) and acrophase determined (mean ± SEM, n = 8, t-test, * p<0.05, versus Control). (C) Peritoneal macrophages were synchronized and mRNA levels of Bmal1 and 18S were assessed every 8h with qRT-PCR in presence or absence of LPS (20ng/ml). Transcript abundance (ΔΔCt) was reported relative to Time 0 in the control group (mean ± SEM, n = 5, one-way ANOVA with Bonferroni post hoc correction, *p<0.05, versus control).
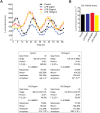
Fig 2. LPS promotes a circadian phase-shift at low concentrations.
Varying doses of LPS (0, 5, 20 and 100ng/ml) were added into the luminescence buffer at Time 0 after serum shock. (A) Left panel: Bioluminescence were recorded every 2h continuing for 68 hours. Right panel: Relative cell numbers were measured via a cell viability assay at the end of the luminescence measurements (mean ± SEM, n = 4, one-way ANOVA, ns versus Control). (B) Oscillation curves were analyzed by cosinor and acrophase compared by one-way Anova with Bonferroni post hoc correction (mean ± SEM, n = 8, *p<0.05, versus control, # p<0.05, versus LPS 5ng/ml).
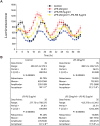
Fig 3. Inhibition of TLR4 reverses the LPS-induced phase-shift.
Peritoneal macrophages were isolated from _mPer2_luciferase transgenic mice and subjected to synchronization via dexamethasone shock. 20ng/ml LPS with or without LPS-RS (5μg/ml) was added to the luminescence buffer at Time 0. (A) Bioluminescence were recorded every 2h continuing for 68 hours. (B) Oscillation curves were analyzed by cosinor and acrophase compared by one-way Anova with Bonferroni post hoc correction (mean ± SEM, n = 8, *p<0.05, _versus_ Control, ns _p_>0.05, versus LPS-RS).
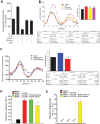
Fig 4. LPS stimulates ROS release from peritoneal macrophage and elevated ROS impairs the function of circadian transcription factors.
(A) Peritoneal macrophages were isolated from _mPer2_luciferase transgenic mice and subjected to different treatments over 24h (LPS 20ng/ml, LPS-RS 5μg/ml, gp91 ds-tat 1μM). Unstimulated or basal superoxide release was monitored using L-012 chemiluminescence (mean ± SEM, n = 5, one-way ANOVA with Bonferroni post hoc correction, *p<0.05, versus Control). (B) Peritoneal macrophages from _mPer2_luciferase mice were subjected to different treatments (LPS 20ng/ml, PEG-SOD, 100U/ml, Tempol 0.4mM) and bioluminescence recorded every 2h for 68 hours (mean ± SEM, n = 5, acrophase were compared via one-way ANOVA with Bonferroni post hoc correction, *p<0.05, versus control. # p<0.05, versus LPS). (C) U2OS _Bma1_luciferase cells were transduced with active or inactive Nox5 adenovirus (15 MOI) and the oscillation of expressed luciferase activity recorded every 2h after serum shock. Oscillation curves were analyzed by cosinor and acrophase compared by one-way Anova with Bonferroni post hoc correction (mean ± SEM, n = 6, *p<0.05, versus Control, # p<0.05, versus Nox5 active). (D) Per1 promoter transactivation was assessed by a dual luciferase assay in transfected COS cells expressing BMAL1, BMAL1+CLOCK in the presence or absence of the ROS generator NOX5 or an inactive NOX5 enzyme (H268Q), (mean ± SEM, n = 5, one-way ANOVA with Bonferroni post hoc correction, *p<0.05, versus Bmal1 alone. # p<0.05, versus Nox5 active). (E) SOD-sensitive superoxide production was monitored by L-012 chemiluminescence. Results are presented as mean ± SEM, n = 6, one-way ANOVA with Bonferroni post hoc correction, *p<0.05, versus Bmal1 alone.
Fig 5. LPS increases NO release from peritoneal macrophage and iNOS-derived NO has no effect on circadian transcription factor activity.
(A) NO release was measured by chemiluminescence detection of NO2− (mean ± SEM, n = 5, one-way ANOVA with Bonferroni post hoc correction, *p<0.05, versus control. # p<0.05, versus LPS). (B) Dual luciferase assay in COS cells expressing the _Per_1 promoter luciferase and BMAL1 and CLOCK or BMAL1, BMAL1+NPAS2+iNOS in the presence or absence of L-NAME (2mM), mean ± SEM, n = 6, one-way ANOVA with Bonferroni post hoc correction, *p<0.05, versus Bmal1 alone). (C) Peritoneal macrophages were synchronized as described and exposed to LPS (20ng/ml) with or without L-NAME (2mM). Bioluminescence was recorded every 2h for 48 hours. Oscillation curves were analyzed by cosinor and acrophase compared by one-way Anova with Bonferroni post hoc correction (mean ± SEM, n = 5, *p<0.05, versus Control, ns versus LPS).
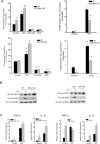
Fig 6. Circadian genes knockout alters the balance ROS and NO release from peritoneal macrophages.
Peritoneal macrophages were isolated from WT mice and circadian gene knockout mice (_Bmal1_-KO mice and _Per_-TKO mice), and cells were subjected to different treatments over 24h. (A) Unstimulated or basal superoxide release was monitored using L-012. NO release was measured by NO-specific chemiluminescence of NO2−. The data was normalized by residual NO2− detected in the presence of L-NAME (mean ± SEM, n = 6, two-way ANOVA with Bonferroni post hoc correction, *p<0.05, versus control. # p<0.05, versus WT). (B) Peritoneal macrophages were isolated from WT or circadian clock knockout mice (Bmal1 KO and Per-TKO), exposed to vehicle or LPS (20ng/ml, 24h) and lysed in Laemmli sample buffer. Cell lysates were subjected to SDS-PAGE and immunoblotted with antibodies to gp91phox (NOX2), iNOS and GAPDH. Results are representative of 3 experiments. (C) Peritoneal macrophages were isolated from WT or circadian clock knockout mice (Bmal1 KO and _Per_-TKO), exposed to vehicle or LPS (20ng/ml, 24h) and lysed in TRIZOL for mRNA extraction. Relative mRNA expression levels of Tnfα and Il-6 were measured by qRT-PCR (ΔΔCt) normalized to GAPDH (mean ± SEM, n = 6, two-way ANOVA with Bonferroni post hoc correction, *p<0.05 versus control, # p<0.05 versus WT).
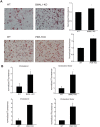
Fig 7. Circadian clock disruption increases LDL uptake by macrophages.
Peritoneal macrophages were isolated from WT, Bmal1 KO or _Per_-TKO mice and cells exposed to oxLDL (50μg/ml) for 72h. (A) Oil red O staining for lipid uptake in macrophages. The extent of staining was quantitated by measurement of fluorescent intensity. (mean ± SEM n = 4, t-test, *p<0.05 versus WT). (B) Measurement of cholesterol ester and free cholesterol content from macrophages. (mean ± SEM n = 4, t-test, *p<0.05 versus WT
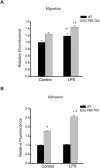
Fig 8. Circadian clock disruption alters the migration and adhesion of macrophages.
(A) The effect of circadian disruption on macrophage migration was evaluated using the Oris Cell Migration Assay. Peritoneal macrophages were isolated from WT and _Per_-TKO mice and subjected to the indicated treatment (LPS 100ng/ml) for 24h, cells stained with calcein AM for 30 min and the fluorescence signal in the migration zone quantified. (mean ± SEM n = 4–5, two-way ANOVA with Bonferroni post hoc correction, *p<0.05 versus control, # p<0.05 versus WT). (B) Fluorescently labeled peritoneal macrophages were incubated with activated adherent human aortic endothelial cells for 15 minutes at 37°C and the degree of cell adhesion assay was quantified (mean ± SEM n = 6, two-way ANOVA with Bonferroni post hoc correction, *p<0.05 versus control, # p<0.05 versus WT).
Similar articles
- BMAL2 promotes eCIRP-induced macrophage endotoxin tolerance.
Zhou M, Aziz M, Li J, Jha A, Ma G, Murao A, Wang P. Zhou M, et al. Front Immunol. 2024 Jun 13;15:1426682. doi: 10.3389/fimmu.2024.1426682. eCollection 2024. Front Immunol. 2024. PMID: 38938563 Free PMC article. - TNFRp55 modulates IL-6 and nitric oxide responses following Yersinia lipopolysaccharide stimulation in peritoneal macrophages.
Eliçabe RJ, Arias JL, Rabinovich GA, Di Genaro MS. Eliçabe RJ, et al. Immunobiology. 2011 Dec;216(12):1322-30. doi: 10.1016/j.imbio.2011.05.009. Epub 2011 May 24. Immunobiology. 2011. PMID: 21802165 - [Optimization of the dosage schedule for sustaining intrinsic biological rhythms].
Koyanagi S. Koyanagi S. Yakugaku Zasshi. 2003 Sep;123(9):789-97. doi: 10.1248/yakushi.123.789. Yakugaku Zasshi. 2003. PMID: 14513770 Review. Japanese. - Circadian Control of Redox Reactions in the Macrophage Inflammatory Response.
O'Siorain JR, Curtis AM. O'Siorain JR, et al. Antioxid Redox Signal. 2022 Oct;37(10-12):664-678. doi: 10.1089/ars.2022.0014. Epub 2022 Mar 29. Antioxid Redox Signal. 2022. PMID: 35166129 Review.
Cited by
- Genetics of flight in spongy moths (Lymantria dispar ssp.): functionally integrated profiling of a complex invasive trait.
Blackburn GS, Keeling CI, Prunier J, Keena MA, Béliveau C, Hamelin R, Havill NP, Hebert FO, Levesque RC, Cusson M, Porth I. Blackburn GS, et al. BMC Genomics. 2024 May 31;25(1):541. doi: 10.1186/s12864-023-09936-8. BMC Genomics. 2024. PMID: 38822259 Free PMC article. - Time is on the Immune System's Side, Yes it is.
Abele SH, Meadows KE, Medeiros D, Silver AC. Abele SH, et al. Yale J Biol Med. 2019 Jun 27;92(2):225-231. eCollection 2019 Jun. Yale J Biol Med. 2019. PMID: 31249483 Free PMC article. Review. - Exploring Clinical Trials to Manage Firefighters' Sleep Quality: A PRISMA Compliant Systematic Review.
Alves S, Vaz J, Fernandes A. Alves S, et al. Int J Environ Res Public Health. 2023 Feb 21;20(5):3862. doi: 10.3390/ijerph20053862. Int J Environ Res Public Health. 2023. PMID: 36900873 Free PMC article. - NOX2 inhibition enables retention of the circadian clock in BV2 microglia and primary macrophages.
Muthukumarasamy I, Buel SM, Hurley JM, Dordick JS. Muthukumarasamy I, et al. Front Immunol. 2023 Feb 6;14:1106515. doi: 10.3389/fimmu.2023.1106515. eCollection 2023. Front Immunol. 2023. PMID: 36814920 Free PMC article. - Molecular Interactions Between Components of the Circadian Clock and the Immune System.
Hergenhan S, Holtkamp S, Scheiermann C. Hergenhan S, et al. J Mol Biol. 2020 May 29;432(12):3700-3713. doi: 10.1016/j.jmb.2019.12.044. Epub 2020 Jan 10. J Mol Biol. 2020. PMID: 31931006 Free PMC article. Review.
References
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975–8. . - PubMed
- Hirota T, Fukada Y. Resetting mechanism of central and peripheral circadian clocks in mammals. Zoolog Sci. 2004;21(4):359–68. . - PubMed
- Zhang Z, Ma F, Zhou F, Chen Y, Wang X, Zhang H, et al. Functional polymorphisms of circadian negative feedback regulation genes are associated with clinical outcome in hepatocellular carcinoma patients receiving radical resection. Med Oncol. 2014;31(12):179 10.1007/s12032-014-0179-1 . - DOI - PubMed
- Zulch KJ, Hossmann V. 24-hour rhythm of human blood pressure. German medical monthly. 1967;12(11):513–8. Epub 1967/11/01. . - PubMed
- Hossmann V, Fitzgerald GA, Dollery CT. Circadian rhythm of baroreflex reactivity and adrenergic vascular response. Cardiovascular research. 1980;14(3):125–9. Epub 1980/03/01. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials