Thermotaxis, circadian rhythms, and TRP channels in Drosophila - PubMed (original) (raw)
Review
Thermotaxis, circadian rhythms, and TRP channels in Drosophila
Andrew Bellemer. Temperature (Austin). 2015.
Abstract
The fruit fly Drosophila melanogaster is a poikilothermic organism that must detect and respond to both fine and coarse changes in environmental temperature in order maintain optimal body temperature, synchronize behavior to daily temperature fluctuations, and to avoid potentially injurious environmental hazards. Members of the Transient Receptor Potential (TRP) family of cation channels are well known for their activation by changes in temperature and their essential roles in sensory transduction in both invertebrates and vertebrates. The Drosophila genome encodes 13 TRP channels, and several of these have key sensory transduction and modulatory functions in allowing larval and adult flies to make fine temperature discriminations to attain optimal body temperature, detect and avoid large environmental temperature fluctuations, and make rapid escape responses to acutely noxious stimuli. Drosophila use multiple, redundant signaling pathways and neural circuits to execute these behaviors in response to both increases and decreases in temperature of varying magnitudes and time scales. A plethora of powerful molecular and genetic tools and the fly's simple, well-characterized nervous system have given Drosophila neurobiologists a powerful platform to study the cellular and molecular mechanisms of TRP channel function and how these mechanisms are conserved in vertebrates, as well as how these channels function within sensorimotor circuits to generate both simple and complex thermosensory behaviors.
Keywords: A1, 1st Antennal Segment; A2, 2nd Antennal Segment; A3, 3rd Antennal Segment; AC, Anterior Cell; AL, Antennal Lobe; AR, Arista; Clk, Clock protein; Cry, Cryptochrome; Cyc, Cycle protein; DN1, DN2, DN3, Dorsal Neuron group 1, 2, 3; Dbt, Double Time protein; Drosophila melanogaster; GFP, Green Fluorescent Protein; GPCR, G Protein-Coupled Receptor; LN, Lateral Neuron; LNd, Dorsal Lateral Neuron; LNv, Ventral Lateral Neuron; LPN, Lateral Posterior Neuron; NEL, Nocifensive Escape Locomotion; PAP, Proximal Antennal Protocerebrum; PDF, Pigment Dispersing Factor; PKD1, Polycistic Kidney Disease 1; PLC, Phospholipase C; Per, Period protein; RNAi, RNA interference; SAC, Sacculus; SLPR, Superior Lateral Protocerebrum; SOG, Suboesophageal Ganglion; TRP channels; TRP, Transient Receptor Potential; TRPA, Transient Receptor Potential, group A (ankyrin repeat); TRPA1; TRPC, Transient Receptor Potential, group C (canonical); TRPL, TRP-Like; TRPM, Transient Receptor Potential, group M (melastatin); TRPP, Transient Receptor Potential, group P (polycystic); TRPV, Transient Receptor Potential, group V (vanilloid); Tim, Timeless protein; VFP, Venus Fluorescent Protein; circadian rhythms; lLNv, Ventral Lateral Neuron, large cell body; mdIV, Multidendritic Neuron, class IV; nociception; sLNv, Ventral Lateral Neuron, small cell body; thermoTRP, thermosensitive TRP channel; thermosensation; thermotaxis.
Figures
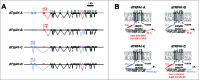
Figure 1.
Schematic representation of dTrpA1 isoforms. (A) Gene structures of known dTrpA1 isoforms. Exons are numbered sequentially starting with the most 5′ exon and irrespective of isoform. The “A” isoform uses a translation start site in exon 3 and is spliced to include exon 12. The “B” isoform uses a translation start site in exon 3 and is spliced to include exon 13. The “C” isoform uses a translation start site in exon 1 and is spliced to include exon 13. The “D” isoform uses a translation start site in exon 1 and is spliced to include exon 12. (B) Protein structures of dTRPA1 protein isoforms. N-terminal regions encoded by exons 1 and 2 are drawn in blue, while N-terminal regions encoded by exon 3 are drawn in red. The TAC region between the ankyrin repeats and first transmembrane domain encoded by exon 12 is drawn in red, while the TRP ankyrin cap (TAC) region encoded by exon 13 is drawn in blue.
Figure 2.
Schematic representation of Painless protein isoforms. The Painlessp103, Painlessp72, and Painlessp60 protein isoforms are schematized with varying numbers of ankyrin repeats in their N-terminal intracellular regions.
Figure 3.
Paradigms for assaying thermosensory behavior in Drosophila larvae and adults. (A) Schematic of a circular assay chamber in which flies or larvae must choose between warm and cool (or room temperature and test temperature) regions. The distribution of individuals between these regions can then be used to calculate and avoidance (or preference) index. (B) Schematic of an assay chamber that can be used to test the distribution of individuals across a temperature gradient. The chamber is heated (or cooled) at one end to produce a temperature gradient and divided into zones. The proportional distribution of flies or larvae across these zones can then be calculated. (C) A standard equation used to generate an avoidance index of flies tested in an experimental chamber that requires them to navigate to either room temperature or a test temperature.
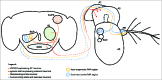
Figure 4.
Central and peripheral neural mechanisms for detecting changes in temperature. The adult Drosophila brain and antenna are schematized here, with colored circles representing neuronal cell bodies, colored lines representing axonal connections, and colored diamonds representing presynaptiC-terminals. Heat-responsive and cool-responsive regions of the proximal antennal protocerebrum (PAP) are schematized as filled ovals with dashed outlines. Heat-sensing neurons of the aristae (AR) connected to the third antennal segment (A3) send projections to the brain that synapse in the heat-sensitive region of the PAP. Cool-sensing neurons of the aristae and sacculus (SAC) send projections to the cool-responsive region of the PAP. Putative _pyrexia-Gal4_-expressing neurons in the second antennal segment (A2) project to the brain and synapse on the dTRPA1-expressing AC neurons (it is important to note that the exact location and identity of these neurons are unknown). The AC neurons project to the heat-responsive region of the PAP, as well as to the suboesophageal ganglion (SOG), the superior lateral protocerebrum (SLPR), and the antennal lobe (AL). Also depicted is the first antennal segment (A1).
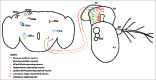
Figure 5.
Central and peripheral neural mechanisms for circadian entrainment to temperature. The adult Drosophila brain and antenna are schematized here, with colored circles representing neuronal cell bodies, colored lines representing axonal connections, and colored diamonds representing presynaptiC-terminals. Shaded areas are used to represent morning and evening oscillators in the circadian circuit. Unidentified _pyrexia-Gal4_-expressing neurons of the second antennal segment (A2) project to the brain and potentially provide input to central circadian pacemakers via unknown synapses. Chordotonal neurons in Johnston's Organ of the second antennal segment and also in the body (not shown) are associated with Pyrexia-expressing cap cells and provide input to the central circadian pacemakers via unknown synapses. The morning oscillator (cream shading) is comprised of 4 of the small ventral lateral neurons (sLNvs) and a subset of the first group of dorsal neurons (DN1s). The evening oscillator is comprised of the fifth sLNv neuron, the dorsal lateral neurons (LNds), and a subset of the DN1s. Cryptochrome functions as the central nervous system photoreceptor of the circadian system and is expressed in the sLNvs, the large ventral lateral neurons (lLNvs), a subset of the LNds, and a subset of the DN1s. dTRPA1 functions as a component of a central nervous system temperature sensor for the circadian system. dTrpA1-Gal4 is expressed in the lateral posterior neurons (LPNs) as well as subsets of the sLNvs, the LNds, the DN1s, the second group of dorsal neurons (DN2s), and the third group of dorsal neurons (DN3s).
Similar articles
- Temperature sensation in Drosophila.
Barbagallo B, Garrity PA. Barbagallo B, et al. Curr Opin Neurobiol. 2015 Oct;34:8-13. doi: 10.1016/j.conb.2015.01.002. Epub 2015 Jan 21. Curr Opin Neurobiol. 2015. PMID: 25616212 Free PMC article. Review. - TRP Channels and Pain.
González-Ramírez R, Chen Y, Liedtke WB, Morales-Lázaro SL. González-Ramírez R, et al. In: Emir TLR, editor. Neurobiology of TRP Channels. Boca Raton (FL): CRC Press/Taylor & Francis; 2017. Chapter 8. In: Emir TLR, editor. Neurobiology of TRP Channels. Boca Raton (FL): CRC Press/Taylor & Francis; 2017. Chapter 8. PMID: 29356491 Free Books & Documents. Review. - Evolutionary conservation and changes in insect TRP channels.
Matsuura H, Sokabe T, Kohno K, Tominaga M, Kadowaki T. Matsuura H, et al. BMC Evol Biol. 2009 Sep 10;9:228. doi: 10.1186/1471-2148-9-228. BMC Evol Biol. 2009. PMID: 19740447 Free PMC article. - Role of Transient Receptor Potential Channels in Acute and Chronic Itch.
Wilson SR, Bautista DM. Wilson SR, et al. In: Carstens E, Akiyama T, editors. Itch: Mechanisms and Treatment. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 16. In: Carstens E, Akiyama T, editors. Itch: Mechanisms and Treatment. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 16. PMID: 24830011 Free Books & Documents. Review. - Drosophila menthol sensitivity and the Precambrian origins of transient receptor potential-dependent chemosensation.
Himmel NJ, Letcher JM, Sakurai A, Gray TR, Benson MN, Cox DN. Himmel NJ, et al. Philos Trans R Soc Lond B Biol Sci. 2019 Nov 11;374(1785):20190369. doi: 10.1098/rstb.2019.0369. Epub 2019 Sep 23. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 31544603 Free PMC article.
Cited by
- The physiological role of TRP channels in sleep and circadian rhythm.
Woodard GE, Rosado JA, Li H. Woodard GE, et al. J Cell Mol Med. 2024 May;28(9):e18274. doi: 10.1111/jcmm.18274. J Cell Mol Med. 2024. PMID: 38676362 Free PMC article. Review. - Hymenopteran-specific TRPA channel from the Texas leaf cutter ant (Atta texana) is heat and cold activated and expression correlates with environmental temperature.
York JM, Taylor TN, LaPotin S, Lu Y, Mueller U. York JM, et al. Insect Sci. 2025 Feb;32(1):301-320. doi: 10.1111/1744-7917.13364. Epub 2024 Apr 11. Insect Sci. 2025. PMID: 38605428 Free PMC article. - RNA-seq analysis reveals TRPC genes to impact an unexpected number of metabolic and regulatory pathways.
Formoso K, Susperreguy S, Freichel M, Birnbaumer L. Formoso K, et al. Sci Rep. 2020 Apr 29;10(1):7227. doi: 10.1038/s41598-020-61177-x. Sci Rep. 2020. PMID: 32350291 Free PMC article. - Thermosensation and Temperature Preference: From Molecules to Neuronal Circuits in Drosophila.
Chiang MH, Lin YC, Wu T, Wu CL. Chiang MH, et al. Cells. 2023 Dec 8;12(24):2792. doi: 10.3390/cells12242792. Cells. 2023. PMID: 38132112 Free PMC article. Review. - When Choice Makes Sense: Menthol Influence on Mating, Oviposition and Fecundity in Drosophila melanogaster.
Abed-Vieillard D, Cortot J. Abed-Vieillard D, et al. Front Integr Neurosci. 2016 Feb 22;10:5. doi: 10.3389/fnint.2016.00005. eCollection 2016. Front Integr Neurosci. 2016. PMID: 26941622 Free PMC article.
References
- Powsner L. The effects of temperature on the durations of the developmental stages of Drosophila melanogaster. Physiol Zool 1935; 8:474–520.
- Delcour J, Lints FA. Environmental and genetic variations of wing size, cell size and cell division rate, inDrosophila melanogaster. Genetica 1966; 37:543-56; http://dx.doi.org/10.1007/BF01547152 - DOI - PubMed
- Kuntz SG, Eisen MB. Drosophila embryogenesis scales uniformly across temperature in developmentally diverse species. PLoS Genet 2014; 10:e1004293; PMID:24762628; http://dx.doi.org/10.1371/journal.pgen.1004293 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials