Prior Binge Ethanol Exposure Potentiates the Microglial Response in a Model of Alcohol-Induced Neurodegeneration - PubMed (original) (raw)
Prior Binge Ethanol Exposure Potentiates the Microglial Response in a Model of Alcohol-Induced Neurodegeneration
Simon Alex Marshall et al. Brain Sci. 2016.
Abstract
Excessive alcohol consumption results in neurodegeneration which some hypothesize is caused by neuroinflammation. One characteristic of neuroinflammation is microglial activation, but it is now well accepted that microglial activation may be pro- or anti-inflammatory. Recent work indicates that the Majchrowicz model of alcohol-induced neurodegeneration results in anti-inflammatory microglia, while intermittent exposure models with lower doses and blood alcohol levels produce microglia with a pro-inflammatory phenotype. To determine the effect of a repeated binge alcohol exposure, rats received two cycles of the four-day Majchrowicz model. One hemisphere was then used to assess microglia via immunohistochemistry and while the other was used for ELISAs of cytokines and growth factors. A single binge ethanol exposure resulted in low-level of microglial activation; however, a second binge potentiated the microglial response. Specifically, double binge rats had greater OX-42 immunoreactivity, increased ionized calcium-binding adapter molecule 1 (Iba-1+) cells, and upregulated tumor necrosis factor-α (TNF-α) compared with the single binge ethanol group. These data indicate that prior ethanol exposure potentiates a subsequent microglia response, which suggests that the initial exposure to alcohol primes microglia. In summary, repeated ethanol exposure, independent of other immune modulatory events, potentiates microglial activity.
Keywords: TNF-alpha; alcohol; alcoholism; cytokines; ethanol; microglia; microglial priming; neurodegeneration.
Figures
Figure 1
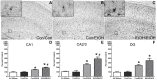
Potentiated Microglial Activation in the Hippocampus by Repeated Ethanol Exposure. OX-42 (CD11b/c) is upregulated in the hippocampus of ethanol-exposed rats as shown in representative photomicrographs of the (A–C) hippocampal dentate gyrus for (B) Con/EtOH and (C) EtOH/EtOH groups compared to (A) controls. Analysis of OX-42 immunoreactivity indicated that the EtOH/EtOH group had significantly more staining than the Con/EtOH group in the: (D) cornu amonis 1 (CA1) and (E) cornu amonis 2/3 (CA2/3) regions but not the (F) dentate gyrus (DG). Data expressed as a percentage of ad libitum control (not shown). Images were taken at 50× magnification with insets at 600× magnification. Scale bar = 200 µm; inset 30 µm. * p < 0.05 compared to ad libitum and Con/Con groups; # p < 0.05 compared to Con/EtOH.
Figure 2
Potentiated Microglial Activation in the Entorhinal Cortex by Repeated Ethanol Exposure. OX-42 (CD11b) is upregulated in the entorhinal cortex of ethanol-exposed rats as shown in representative photomicrographs of the (A–C) entorhinal cortex for (B) Con/EtOH and (C) EtOH/EtOH groups compared to (A) controls. Analysis of OX-42 immunoreactivity indicated that the EtOH/EtOH group had significantly more positive pixels than the Con/EtOH group in the (D) entorhinal cortex. Data expressed as a percentage of ad libitum control (not shown). Images were taken at 200× magnification with insets at 600× magnification. Scale bar = 100 µm; inset 30 µm. * p < 0.05 compared to ad libitum and Con/Con groups; # p < 0.05 compared to Con/EtOH.
Figure 3
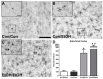
Lack of ED-1 Positive Cells. ED-1 was not visible in the parenchyma of the (A–C) hippocampus or (D–F) entorhinal cortex as seen in representative photomicrographs in (A,D) controls, (B,E) Con/EtOH (C,F) or EtOH/EtOH groups. ED-1 positive cells could be seen along the hippocampal fissure and blood vessels as shown in the inset of B. Scale bars = 200 µm.
Figure 4
Lack of OX-6 Positive Cells. No OX-6 positive cells were observed regardless of treatment, except in one EtOH/EtOH rat as shown in representative photomicrographs of the (A–C) hippocampus or (E–H) entorhinal cortex in (A,E) controls, (B,F) Con/EtOH (C,G) or EtOH/EtOH groups. OX-6 positive cells could be seen along blood vessels as shown in the inset of B. One EtOH/EtOH animal showed upregulation of OX-6 in both the (D) hippocampus and (H) entorhinal cortex. Scale bars = 200 µm.
Figure 5
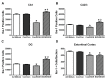
Microglial Cell Counts Differentially Altered by Ethanol Experience. Stereological estimates indicate an increase in the number of microglia in the EtOH/EtOH group in the (A) cornu amonis 1 (CA1), (B) cornu amonis 2/3 (CA2/3), and (C) dentate gyrus (DG) compared with all other groups. However, the number of microglia in the Con/EtOH group was decreased throughout the hippocampus. In the (D) entorhinal cortex, microglia were decreased in both the Con/EtOH and EtOH/EtOH groups compared to both the ad libitum and Con/Con groups. * p < 0.05 compared to ad libitum and Con/Con group; # p < 0.05 versus Con/EtOH.
Figure 6
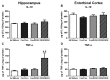
Increased TNF-α in EtOH/EtOH Group. Concentrations of (A,B) interleukin-10 (IL-10) and (C,D) tumor necrosis factor-α (TNF-α) were determined by ELISA in both the hippocampus (A,C) and entorhinal cortex (B,D). No change in IL-10 was measured in either the hippocampus or the entorhinal cortex, but at least a 2.7-fold increase in TNF-α was measured in the (C) hippocampus in the EtOH/EtOH group compared with all other groups. However, no change in TNF-α was seen in the (D) entorhinal cortex. * p < 0.05 compared to ad libitum and Con/Con groups; # p < 0.05 compared to Con/EtOH.
Figure 7
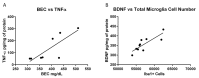
Correlations of Cytokines: (A) A positive correlation between blood ethanol concentration (BEC) and tumor necrosis factor-α (TNF-α) concentration. Animals with BECs over 400 mg/dL appear to have an increase in TNF-α. (B) A positive correlation between hippocampal estimates of microglia number and brain derived neurotrophic factor (BDNF) concentrations in the Con/EtOH group. A decline in the number of microglia cells correlated with decreases in BDNF concentrations.
Figure 8
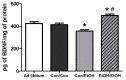
Ethanol Experience-Contingent Effects on BDNF. Concentrations of brain derived neurotrophic factor (BDNF) were determined by ELISA in the hippocampus. BDNF was decreased by approximately 15% in Con/EtOH treated animals compared with Con/Con or ad libitum groups but increased by 20% in the EtOH/EtOH group. * p < 0.05 vs. ad libitum and Con/Con groups; # p < 0.05 vs. to Con/EtOH.
Similar articles
- Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype.
Marshall SA, McClain JA, Kelso ML, Hopkins DM, Pauly JR, Nixon K. Marshall SA, et al. Neurobiol Dis. 2013 Jun;54:239-51. doi: 10.1016/j.nbd.2012.12.016. Epub 2013 Jan 8. Neurobiol Dis. 2013. PMID: 23313316 Free PMC article. - Repetitive binge-like consumption based on the Drinking-in-the-Dark model alters the microglial population in the mouse hippocampus.
Nelson JC, Greengrove E, Nwachukwu KN, Grifasi IR, Marshall SA. Nelson JC, et al. J Integr Neurosci. 2021 Dec 30;20(4):933-943. doi: 10.31083/j.jin2004094. J Integr Neurosci. 2021. PMID: 34997716 Free PMC article. - A comparison of hippocampal microglial responses in aged and young rodents following dependent and non-dependent binge drinking.
Grifasi IR, Evans WA, Rexha AD, Sako LW, Marshall SA. Grifasi IR, et al. Int Rev Neurobiol. 2019;148:305-343. doi: 10.1016/bs.irn.2019.10.018. Epub 2019 Nov 1. Int Rev Neurobiol. 2019. PMID: 31733666 Free PMC article. - Role of microglia in ethanol-induced neurodegenerative disease: Pathological and behavioral dysfunction at different developmental stages.
Yang JY, Xue X, Tian H, Wang XX, Dong YX, Wang F, Zhao YN, Yao XC, Cui W, Wu CF. Yang JY, et al. Pharmacol Ther. 2014 Dec;144(3):321-37. doi: 10.1016/j.pharmthera.2014.07.002. Epub 2014 Jul 10. Pharmacol Ther. 2014. PMID: 25017304 Review. - Ethanol-Induced Neurodegeneration and Glial Activation in the Developing Brain.
Saito M, Chakraborty G, Hui M, Masiello K, Saito M. Saito M, et al. Brain Sci. 2016 Aug 16;6(3):31. doi: 10.3390/brainsci6030031. Brain Sci. 2016. PMID: 27537918 Free PMC article. Review.
Cited by
- Polygenic risk for alcohol use disorder affects cellular responses to ethanol exposure in a human microglial cell model.
Li X, Liu J, Boreland AJ, Kapadia S, Zhang S, Stillitano AC, Abbo Y, Clark L, Lai D, Liu Y, Barr PB, Meyers JL, Kamarajan C, Kuang W, Agrawal A, Slesinger PA, Dick D, Salvatore J, Tischfield J, Duan J, Edenberg HJ, Kreimer A, Hart RP, Pang ZP. Li X, et al. Sci Adv. 2024 Nov 8;10(45):eado5820. doi: 10.1126/sciadv.ado5820. Epub 2024 Nov 8. Sci Adv. 2024. PMID: 39514655 Free PMC article. - The impact of abstinence from chronic alcohol consumption on the mouse striatal proteome: sex and subregion-specific differences.
Duffus BM, Haggerty DL, Doud EH, Mosley AL, Yamamoto BK, Atwood BK. Duffus BM, et al. Front Pharmacol. 2024 Jun 3;15:1405446. doi: 10.3389/fphar.2024.1405446. eCollection 2024. Front Pharmacol. 2024. PMID: 38887549 Free PMC article. - Alcohol binge drinking induces downregulation of blood-brain barrier proteins in the rat frontal cortex -but not in the hippocampus- that is not prevented by OEA pretreatment.
Rodríguez-González A, Moya M, Rodríguez de Fonseca F, Gómez de Heras R, Orio L. Rodríguez-González A, et al. Adv Drug Alcohol Res. 2023 Feb 24;3:11091. doi: 10.3389/adar.2023.11091. eCollection 2023. Adv Drug Alcohol Res. 2023. PMID: 38389819 Free PMC article. - Microglia activity in the human basal ganglia is altered in alcohol use disorder and reversed with remission from alcohol.
Rasool AE, Furlong T, Prasad AA. Rasool AE, et al. Addict Biol. 2024 Feb;29(2):e13374. doi: 10.1111/adb.13374. Addict Biol. 2024. PMID: 38380734 Free PMC article. - Neuroimmune Activation and Microglia Reactivity in Female Rats Following Alcohol Dependence.
Melbourne JK, Wooden JI, Carlson ER, Anasooya Shaji C, Nixon K. Melbourne JK, et al. Int J Mol Sci. 2024 Jan 28;25(3):1603. doi: 10.3390/ijms25031603. Int J Mol Sci. 2024. PMID: 38338883 Free PMC article.
References
- Grant B.F., Saha T.D., Ruan W.J., Goldstein R.B., Chou S.P., Jung J., Zhang H., Smith S.M., Pickering R.P., Huang B., et al. Epidemiology of DSM-5 drug use disorder: Results from the national epidemiologic survey on alcohol and related conditions-III. JAMA Psychiatry. 2016;73:39–47. doi: 10.1001/jamapsychiatry.2015.2132. - DOI - PMC - PubMed
- Pfefferbaum A., Lim K.O., Zipursky R.B., Mathalon D.H., Rosenbloom M.J., Lane B., Ha C.N., Sullivan E.V. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: A quantitative MRI study. Alcohol Clin. Exp. Res. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. - DOI - PubMed
Grants and funding
- F31 AA023459/AA/NIAAA NIH HHS/United States
- K12 GM000678/GM/NIGMS NIH HHS/United States
- R01 AA016959/AA/NIAAA NIH HHS/United States
- T32 DA016176/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources