Cytokine responses and epithelial function in the intestinal mucosa - PubMed (original) (raw)
Review
Cytokine responses and epithelial function in the intestinal mucosa
Joseph C Onyiah et al. Cell Mol Life Sci. 2016 Nov.
Abstract
Inflammatory diseases of mucosal organs are significantly influenced by the microenvironment in which they reside. Cytokines found within this microenvironment contribute significantly to endpoint functions of the mucosa. Studies dating back to the 1990s have revealed that epithelial cells are both a source as well as a target for numerous cytokines and that such signaling can substantially influence the outcome of mucosal disease, such as inflammatory bowel disease. Here, we will review literature regarding intestinal epithelial cells as sources and responders to cytokines found in the intestinal milieu. These studies highlight the dynamic nature of these pathways and lend insight into the complexity of treating mucosal inflammation.
Keywords: Colitis; Epithelium; Inflammation; Leukocyte; Mucosa; Murine model.
Conflict of interest statement
The authors declare no financial interests in any of the work submitted here.
Figures
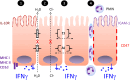
Fig. 1
IFN-gamma as a model cytokine signaling hub in the intestinal mucosa: 1 IFN-gamma signaling through basolaterally localized receptors induces the expression of immune regulatory components including MHC class I and II, CD1d as well as the apical IL-10R. 2 Through the down-regulation of CFTR and multiple ion channels in the epithelial membrane, IFN-gamma abrogates electrogenic chloride secretion and associated water transport (see text for details). 3 IFN-gamma signaling increases epithelial permeability through the regulation of tight junction (TJ) and adherens junction (AJ) component expression. 4 IFN-gamma signaling induces the apical expression of ICAM-1 and the basolateral expression of CD47. Together this allows migration and apical localization of neutrophils (PMN)
Similar articles
- Neutrophils and the inflammatory tissue microenvironment in the mucosa.
Campbell EL, Kao DJ, Colgan SP. Campbell EL, et al. Immunol Rev. 2016 Sep;273(1):112-20. doi: 10.1111/imr.12456. Immunol Rev. 2016. PMID: 27558331 Free PMC article. Review. - Activation of Epithelial Signal Transducer and Activator of Transcription 1 by Interleukin 28 Controls Mucosal Healing in Mice With Colitis and Is Increased in Mucosa of Patients With Inflammatory Bowel Disease.
Chiriac MT, Buchen B, Wandersee A, Hundorfean G, Günther C, Bourjau Y, Doyle SE, Frey B, Ekici AB, Büttner C, Weigmann B, Atreya R, Wirtz S, Becker C, Siebler J, Neurath MF. Chiriac MT, et al. Gastroenterology. 2017 Jul;153(1):123-138.e8. doi: 10.1053/j.gastro.2017.03.015. Epub 2017 Mar 23. Gastroenterology. 2017. PMID: 28342759 - IL-36R signalling activates intestinal epithelial cells and fibroblasts and promotes mucosal healing in vivo.
Scheibe K, Backert I, Wirtz S, Hueber A, Schett G, Vieth M, Probst HC, Bopp T, Neurath MF, Neufert C. Scheibe K, et al. Gut. 2017 May;66(5):823-838. doi: 10.1136/gutjnl-2015-310374. Epub 2016 Jan 18. Gut. 2017. PMID: 26783184 - IFN-γ-mediated induction of an apical IL-10 receptor on polarized intestinal epithelia.
Kominsky DJ, Campbell EL, Ehrentraut SF, Wilson KE, Kelly CJ, Glover LE, Collins CB, Bayless AJ, Saeedi B, Dobrinskikh E, Bowers BE, MacManus CF, Müller W, Colgan SP, Bruder D. Kominsky DJ, et al. J Immunol. 2014 Feb 1;192(3):1267-76. doi: 10.4049/jimmunol.1301757. Epub 2013 Dec 23. J Immunol. 2014. PMID: 24367025 Free PMC article.
Cited by
- Development of a Gut-On-A-Chip Model for High Throughput Disease Modeling and Drug Discovery.
Beaurivage C, Naumovska E, Chang YX, Elstak ED, Nicolas A, Wouters H, van Moolenbroek G, Lanz HL, Trietsch SJ, Joore J, Vulto P, Janssen RAJ, Erdmann KS, Stallen J, Kurek D. Beaurivage C, et al. Int J Mol Sci. 2019 Nov 12;20(22):5661. doi: 10.3390/ijms20225661. Int J Mol Sci. 2019. PMID: 31726729 Free PMC article. - Toll-like Interleukin -1 Receptor Regulator (TILRR) Protein, a Major Modulator of Inflammation, is Expressed in Normal Human and Macaque Tissues and PBMCs.
Kashem MA, Li L, Yuan XY, Plummer FA, Luo M. Kashem MA, et al. J Inflamm Res. 2022 May 12;15:2925-2937. doi: 10.2147/JIR.S357866. eCollection 2022. J Inflamm Res. 2022. PMID: 35592073 Free PMC article. - The Influence of Heat-Killed Enterococcus faecium BGPAS1-3 on the Tight Junction Protein Expression and Immune Function in Differentiated Caco-2 Cells Infected With Listeria monocytogenes ATCC 19111.
Popović N, Djokić J, Brdarić E, Dinić M, Terzić-Vidojević A, Golić N, Veljović K. Popović N, et al. Front Microbiol. 2019 Mar 5;10:412. doi: 10.3389/fmicb.2019.00412. eCollection 2019. Front Microbiol. 2019. PMID: 30891021 Free PMC article. - A Central Role for Heme Oxygenase-1 in the Control of Intestinal Epithelial Chemokine Expression.
Onyiah JC, Schaefer REM, Colgan SP. Onyiah JC, et al. J Innate Immun. 2018;10(3):228-238. doi: 10.1159/000488914. Epub 2018 May 23. J Innate Immun. 2018. PMID: 29791903 Free PMC article. - 3D bioengineered tissue model of the large intestine to study inflammatory bowel disease.
Roh TT, Chen Y, Paul HT, Guo C, Kaplan DL. Roh TT, et al. Biomaterials. 2019 Dec;225:119517. doi: 10.1016/j.biomaterials.2019.119517. Epub 2019 Sep 25. Biomaterials. 2019. PMID: 31580968 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK050189/DK/NIDDK NIH HHS/United States
- I01 BX002182/BX/BLRD VA/United States
- R29 DK050189/DK/NIDDK NIH HHS/United States
- R37 DK050189/DK/NIDDK NIH HHS/United States
- R01 DK095491/DK/NIDDK NIH HHS/United States
- R01 DK104713/DK/NIDDK NIH HHS/United States
- R01 DK103712/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources