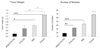iRGD peptide conjugation potentiates intraperitoneal tumor delivery of paclitaxel with polymersomes - PubMed (original) (raw)
iRGD peptide conjugation potentiates intraperitoneal tumor delivery of paclitaxel with polymersomes
Lorena Simón-Gracia et al. Biomaterials. 2016 Oct.
Abstract
Polymersomes are versatile nanoscale vesicles that can be used for cytoplasmic delivery of payloads. Recently, we demonstrated that pH-sensitive polymersomes exhibit an intrinsic selectivity towards intraperitoneal tumor lesions. A tumor homing peptide, iRGD, harbors a cryptic C-end Rule (CendR) motif that is responsible for neuropilin-1 (NRP-1) binding and for triggering extravasation and tumor penetration of the peptide. iRGD functionalization increases tumor selectivity and therapeutic efficacy of systemic drug-loaded nanoparticles in many tumor models. Here we studied whether intraperitoneally administered paclitaxel-loaded iRGD-polymersomes show improved efficacy in the treatment of peritoneal carcinomatosis. First, we demonstrated that the pH-sensitive polymersomes functionalized with RPARPAR (a prototypic CendR peptide) or iRGD internalize in the cells that express NRP-1, and that internalized polymersomes release their cargo inside the cytosol. CendR-targeted polymersomes loaded with paclitaxel were more cytotoxic on NRP-1-positive cells than on NRP-1-negative cells. In mice bearing peritoneal tumors of gastric (MKN-45P) or colon (CT26) origin, intraperitoneally administered RPARPAR and iRGD-polymersomes showed higher tumor-selective accumulation and penetration than untargeted polymersomes. Finally, iRGD-polymersomes loaded with paclitaxel showed improved efficacy in peritoneal tumor growth inhibition and in suppression of local dissemination compared to the pristine paclitaxel-polymersomes or Abraxane. Our study demonstrates that iRGD-functionalization improves efficacy of paclitaxel-polymersomes for intraperitoneal treatment of peritoneal carcinomatosis.
Keywords: NRP-1; Paclitaxel; Peritoneal carcinomatosis; Polymersomes; Tumor penetrating peptides; iRGD.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
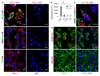
Fig. 1. Receptor binding, cellular internalization, and cargo release of peptide-targeted polymersomes.
(A) Fluorescence confocal imaging of PPC-1 or M21 cells incubated with 0.5 mg/mL of R-FAM-PS, iRGD-FAM-PS or FAM-PS for 1 h. The cells were stained with DAPI and anti-NRP1. Green: polymersomes; red: NRP-1; blue: DAPI. Scale bars: 20 μm. Representative fields from multiple areas of cultured cells from three independent experiments are shown. (B) Binding of R-PS or PS labeled with Rhodamine to recombinant NRP-1 (b1b2 NRP-1) or a control protein, p32, after 1 h of incubation. For this assay, 0.5 mg/mL of polymersome samples were used. Y axis is the polymersome fluorescence in arbitrary units (A.U.). N = 3; statistical analysis was performed by one-way ANOVA; error bars, mean + SEM, *p < 0.05. (C) Fluorescence confocal imaging of PPC-1 cells incubated with 0.5 mg/mL of R-FAM-PS loaded with Rho for one hour. The cells were stained with DAPI. Green: polymersomes; red: Rho; blue: DAPI. Scale bar: 20 μm. Representative fields from multiple areas of cultured cells from three independent experiments are shown. (D) Fluorescence confocal imaging of PPC-1 cells incubated with 0.5 mg/mL of R-PS or PS loaded with DOX for one hour. The cells were stained with DAPI. Green: NRP-1; red: DOX; blue: DAPI. Scale bars: 20 μm. Representative fields from multiple areas of cultured cells from three independent experiments are shown. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2. Effect of paclitaxel-loaded polymersomes on survival of cells with different NRP-1 expression status.
Growth rate dynamics of cultured PPC-1 and M21 cells after addition of the R-PS-PTX, iRGD-PS-PTX, or PS-PTX, and ABX at 100 nM PTX concentration, measured using the xCELLigence® real-time cell analyzer that allows continuous quantitative monitoring of attached cells. 100% viability corresponds to untreated cells. Each data point represents the average of three samples. Error bars, mean ± SEM.
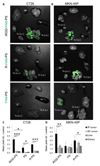
Fig. 3. In vivo biodistribution of IP-administered polymersomes.
(A) Mice bearing dual IP and s.c. MKN-45P or CT26 tumors were IP injected with 0.5 mg of iRGD-FAM-PS, R-FAM-PS, or FAM-PS, and after 4 h the tumors and organs of interest were excised and fluorescent signal was imaged by Illumatool (Lightools Research, CA). Representative compound fluorescent and bright-field images from three independent experiments are shown. He, heart; Lu, lung; Sp, spleen; Ki, kidney; Li, liver; Br, brain, Tu, tumor. (B) Quantification of the fluorescent signal in tumors and control organs by the Image J software. N ≥ 3 mice; statistical analysis was performed by one-way ANOVA; error bars, mean + SEM; ***p < 0.001, **p < 0.01, *p < 0.05.
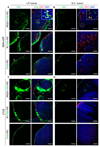
Fig. 4. Confocal imaging of polymersomes in tumor tissue.
(A) Fluorescence confocal images of tissue sections prepared from IP and s.c. MKN-45P and CT26 tumors collected 4 h after IP injection of polymersomes. Representative images from 3 independent experiments are shown. Green: polymersomes; red: CD31 (blood vessels); blue: DAPI. Scale bars: 250 μm. Arrows point to polymersomes co-localizing with blood vessels; arrowheads point to PS in tumor periphery. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5. Experimental therapy of tumor mice.
Mice bearing disseminated peritoneal MKN-45P tumors were injected IP every other day during two weeks with indicated formulations (cumulative dose of the treatment: 7 mg PTX/Kg). The tumor weight (weight of large peritoneal tumors combined with small peritoneal tumor nodules) and number of peritoneal tumor nodules after treatment are shown. N = 8 mice in each group; statistical analysis was performed by one-way ANOVA; error bars, mean + SEM; ***p < 0.001, **p < 0.01, *p < 0.05.
Similar articles
- Co-Administration Of iRGD Enhances Tumor-Targeted Delivery And Anti-Tumor Effects Of Paclitaxel-Loaded PLGA Nanoparticles For Colorectal Cancer Treatment.
Zhong Y, Su T, Shi Q, Feng Y, Tao Z, Huang Q, Li L, Hu L, Li S, Tan H, Liu S, Yang H. Zhong Y, et al. Int J Nanomedicine. 2019 Nov 1;14:8543-8560. doi: 10.2147/IJN.S219820. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31802868 Free PMC article. - Paclitaxel-Loaded Polymersomes for Enhanced Intraperitoneal Chemotherapy.
Simón-Gracia L, Hunt H, Scodeller PD, Gaitzsch J, Braun GB, Willmore AM, Ruoslahti E, Battaglia G, Teesalu T. Simón-Gracia L, et al. Mol Cancer Ther. 2016 Apr;15(4):670-9. doi: 10.1158/1535-7163.MCT-15-0713-T. Epub 2016 Feb 15. Mol Cancer Ther. 2016. PMID: 26880267 Free PMC article. - Targeted Modification of the Cationic Anticancer Peptide HPRP-A1 with iRGD To Improve Specificity, Penetration, and Tumor-Tissue Accumulation.
Hu C, Huang Y, Chen Y. Hu C, et al. Mol Pharm. 2019 Feb 4;16(2):561-572. doi: 10.1021/acs.molpharmaceut.8b00854. Epub 2019 Jan 14. Mol Pharm. 2019. PMID: 30592418 - Recent advances in the tumor-penetrating peptide internalizing RGD for cancer treatment and diagnosis.
Qian J, Zhou S, Lin P, Lei J, Zheng S, Xu W, Wang Y, Gao Z, Yang J. Qian J, et al. Drug Dev Res. 2023 Jun;84(4):654-670. doi: 10.1002/ddr.22056. Epub 2023 Apr 5. Drug Dev Res. 2023. PMID: 36959702 Review. - iRGD Peptide as a Tumor-Penetrating Enhancer for Tumor-Targeted Drug Delivery.
Kang S, Lee S, Park S. Kang S, et al. Polymers (Basel). 2020 Aug 24;12(9):1906. doi: 10.3390/polym12091906. Polymers (Basel). 2020. PMID: 32847045 Free PMC article. Review.
Cited by
- Pro-Tumorigenic Macrophage Infiltration in Oral Squamous Cell Carcinoma and Possible Macrophage-Aimed Therapeutic Interventions.
Bruna F, Scodeller P. Bruna F, et al. Front Oncol. 2021 May 10;11:675664. doi: 10.3389/fonc.2021.675664. eCollection 2021. Front Oncol. 2021. PMID: 34041037 Free PMC article. Review. - Co-Administration Of iRGD Enhances Tumor-Targeted Delivery And Anti-Tumor Effects Of Paclitaxel-Loaded PLGA Nanoparticles For Colorectal Cancer Treatment.
Zhong Y, Su T, Shi Q, Feng Y, Tao Z, Huang Q, Li L, Hu L, Li S, Tan H, Liu S, Yang H. Zhong Y, et al. Int J Nanomedicine. 2019 Nov 1;14:8543-8560. doi: 10.2147/IJN.S219820. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31802868 Free PMC article. - Paclitaxel-Loaded Cationic Fluid Lipid Nanodiscs and Liposomes with Brush-Conformation PEG Chains Penetrate Breast Tumors and Trigger Caspase-3 Activation.
Simón-Gracia L, Scodeller P, Fisher WS, Sidorenko V, Steffes VM, Ewert KK, Safinya CR, Teesalu T. Simón-Gracia L, et al. ACS Appl Mater Interfaces. 2022 Dec 28;14(51):56613-56622. doi: 10.1021/acsami.2c17961. Epub 2022 Dec 15. ACS Appl Mater Interfaces. 2022. PMID: 36521233 Free PMC article. - Localized chemotherapy approaches and advanced drug delivery strategies: a step forward in the treatment of peritoneal carcinomatosis from ovarian cancer.
Breusa S, Zilio S, Catania G, Bakrin N, Kryza D, Lollo G. Breusa S, et al. Front Oncol. 2023 May 23;13:1125868. doi: 10.3389/fonc.2023.1125868. eCollection 2023. Front Oncol. 2023. PMID: 37287910 Free PMC article. Review. - In Silico and In Vivo Studies of a Tumor-Penetrating and Interfering Peptide with Antitumoral Effect on Xenograft Models of Breast Cancer.
Marin GH, Murail S, Andrini L, Garcia M, Loisel S, Tuffery P, Rebollo A. Marin GH, et al. Pharmaceutics. 2023 Apr 7;15(4):1180. doi: 10.3390/pharmaceutics15041180. Pharmaceutics. 2023. PMID: 37111665 Free PMC article.
References
- Intraperitoneal Cancer Therapy: Principles and Practice. CRC Press Book; (n.d.). https://www.crcpress.com/Intraperitoneal-Cancer-Therapy-Principles-and-P... (accessed 04.24.16).
- Eskander RN. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in epithelial ovarian cancer: state of the art. World J Obstet Gynecol. 2013;2:94. doi: 10.5317/wjog.v2.i4.94. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- Wellcome Trust/United Kingdom
- P30 CA030199/CA/NCI NIH HHS/United States
- R01 CA152327/CA/NCI NIH HHS/United States
- R01 CA167174/CA/NCI NIH HHS/United States
- WT095077MA/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous