Autophagy provides metabolic substrates to maintain energy charge and nucleotide pools in Ras-driven lung cancer cells - PubMed (original) (raw)
Autophagy provides metabolic substrates to maintain energy charge and nucleotide pools in Ras-driven lung cancer cells
Jessie Yanxiang Guo et al. Genes Dev. 2016.
Abstract
Autophagy degrades and is thought to recycle proteins, other macromolecules, and organelles. In genetically engineered mouse models (GEMMs) for Kras-driven lung cancer, autophagy prevents the accumulation of defective mitochondria and promotes malignancy. Autophagy-deficient tumor-derived cell lines are respiration-impaired and starvation-sensitive. However, to what extent their sensitivity to starvation arises from defective mitochondria or an impaired supply of metabolic substrates remains unclear. Here, we sequenced the mitochondrial genomes of wild-type or autophagy-deficient (Atg7(-/-)) Kras-driven lung tumors. Although Atg7 deletion resulted in increased mitochondrial mutations, there were too few nonsynonymous mutations to cause generalized mitochondrial dysfunction. In contrast, pulse-chase studies with isotope-labeled nutrients revealed impaired mitochondrial substrate supply during starvation of the autophagy-deficient cells. This was associated with increased reactive oxygen species (ROS), lower energy charge, and a dramatic drop in total nucleotide pools. While starvation survival of the autophagy-deficient cells was not rescued by the general antioxidant N-acetyl-cysteine, it was fully rescued by glutamine or glutamate (both amino acids that feed the TCA cycle and nucleotide synthesis) or nucleosides. Thus, maintenance of nucleotide pools is a critical challenge for starving Kras-driven tumor cells. By providing bioenergetic and biosynthetic substrates, autophagy supports nucleotide pools and thereby starvation survival.
Keywords: ROS; Ras-driven cancer; amino acid; autophagy; energy charge; mitochondrial metabolism; nucleotide.
© 2016 Guo et al.; Published by Cold Spring Harbor Laboratory Press.
Figures
Figure 1.
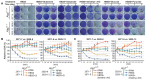
Autophagy mediates substrate recycling in starvation. (A) Schematic of 13C and15N tracer studies. (B) The schematic chart shows different metabolite recycling distributions between Atg7+/+ and _Atg7_−/− TDCLs in starvation. (R3) The time point after cells were cultured in unlabeled RPMI medium for 3 h; (H4) the time point after 4 h of HBSS to induce degradation of intracellular components for recycling. (C) 13C and 15N tracing shows substrates of autophagy-mediated recycling in _Kras_-driven TDCLs in starvation. _t_-test, false discovery rate [FDR]-adjusted _P_-value < 0.05. Red indicates TCA cycle intermediates and TCA-derived amino acids. The error bar indicates ±SEM. n = 3. (D) Pool sizes of metabolites identified from 1C in nutrient-rich (RPMI) and nutrient-depleted (HBSS) conditions. (S7P) Sedoheptulose 7-phosphate. The error bar indicates ±SEM. n = 3. (**) P < 0.01; (***) P < 0.001, _t_-test.
Figure 2.
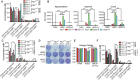
Glutamine and its derivatives sustain mitochondria metabolism to support _Kras_-driven TDCL survival in starvation. (A) Clonogenic survival assay of Atg7 wild-type and _Atg7-_deficient cells in HBSS, HBSS supplemented with 2 mM glutamine, 2 mM glutamate, 1 mM dimethyl-α-KG, 2 g/L glucose, or 1 mM sodium pyruvate. Treated cells were recovered for 3 d in RPMI medium at days 1–3 after starvation. (B) Oxygen consumption rate (OCR) of Atg7+/+ and _Atg7_−/− TDCLs in RPMI, HBSS, and HBSS with 2 mM glutamine (Q) supplementation conditions. The error bar indicates ±SEM. n = 4. (C) OCR of Atg7+/+ or _Atg7_−/− TDCLs in RPMI, HBSS, and HBSS with 1 mM dimethyl-α-KG supplementation conditions. The error bar indicates ±SEM. n = 4.
Figure 3.
Autophagy maintains the redox state of _Kras_-driven TDCLs in starvation. (A) Schematic of carbon atom (circles) transitions and tracers used to detect glutamine flux to TCA cycle intermediates. (B) [U13C5]-Gln flux to TCA cycle intermediates of _Kras_-driven TDCLs in HBSS. The error bar indicates ±SEM. n = 3. (**) P < 0.01; (***) P < 0.001, _t_-test. (C) Glutamine uptake and ammonium secretion rates of TDCLs in HBSS. The error bar indicates ±SEM. n = 3. (**) P < 0.01, _t_-test. (D) The schematic of carbon atom (circles) transitions and tracers shows the metabolic pathway for citrate (M+6) generation from [U13C5]-Gln. (E) ROS measured by 2′-7′-dichlorodihydrofluorescene diacetate (DCFDA) fluorescence in Atg7 wild-type and _Atg7-_deficient cells in RPMI and 4-h HBSS conditions. (F) ROS measured by DCFDA fluorescence shows that 2 mM glutamine supplementation reduced starvation-induced ROS production in _Atg7-_deficient tumor cells. (G) ROS measured by DCFDA fluorescence shows that 0.5 mM N-acetyl-L-cysteine (NAC) supplementation reduced starvation-induced ROS production in _Atg7−/−-_deficient tumor cells. (H) Clonogenic survival assays show that 0.5 mM NAC supplementation did not rescue _Atg7_-deficient cell death in starvation.
Figure 4.
Autophagy sustains energy homeostasis of _Kras_-driven tumor cells in starvation. (A) Energy charge of Atg7+/+ or _Atg7_−/− TDCLs in RPMI and HBSS. The error bar indicates ±SEM (n = 3). (***) P < 0.001, _t_-test. (B) Concentration of adenosine phosphates (ATP, ADP, and AMP) of Atg7 wild-type and _Atg7-_deficient tumor cells in RPMI and HBSS. The error bar indicates ±SEM. n = 3. (**) P < 0.01; (***) P < 0.001, _t_-test. (C) Western blot for pAMPK, total AMPK, and β-actin of Atg7 wild-type and _Atg7-_deficient tumor cells. (D) The level of adenosine phosphates (ATP, ADP, and AMP) in Atg7 wild-type and _Atg7-_deficient tumor cells in HBSS or HBSS supplemented with 2 mM glutamine. The error bar indicates ±SEM. n = 3. (***) P < 0.001, _t_-test. (E) The energy charge of Atg7+/+ and _Atg7_−/− TDCLs in HBSS without or with 2 mM glutamine supplementation. The error bar indicates ±SEM. n = 3. (***) P < 0.001, _t_-test.
Figure 5.
Autophagy sustains nucleotide pools in starvation. (A) The concentration of nucleoside phosphates in Atg7 wild-type and _Atg7-_deficient tumor cells in RPMI and HBSS (4 h). The error bar indicates ±SEM. n = 3. (***) P < 0.001, _t_-test. (B) The level of nucleotide degradation products in Atg7+/+ and _Atg7_−/− TDCLs in RPMI and HBSS (4 h) without or with glutamine supplementation. The error bar indicates ±SEM. n = 3. (***) P < 0.001, _t_-test. (C) The concentration of nucleoside phosphates in Atg7 wild-type and _Atg7-_deficient tumor cells in HBSS (4 h) without or with 2 mM glutamine supplementation. (D) The clonogenic survival assay shows that nucleoside supplementation (2 mM each) rescued starvation-induced _Atg7_-deficient cell death. Treated cells were recovered for 3 d by replacing HBSS with normal RPMI medium after 1 d of starvation. (U) Uridine; (A) adenosine; (G) guanosine; (I) inosine. (E) The energy charge of Atg7+/+ and _Atg7_−/− TDCLs after HBSS (4 h) without or with nucleoside supplementation (2 mM each). (F) The concentration of nucleoside phosphates in _Kras_-driven tumor cells after HBSS (4 h) without or with nucleoside supplementation (2 mM each).
Figure 6.
Mechanisms underlying rescue of starved _Atg7_-deficient tumor cells by glutamine or nucleosides. (A) Schematic of nucleotide degradation and ribose salvage for energy generation. (B) The level of substrates from nucleotide degradation in HBSS (4 h) in the absence or presence of nucleoside supplementation (2 mM each). The error bar indicates ±SEM. n = 3. (***) P < 0.001, _t_-test. (C) Nucleoside addition in HBSS partially rescues levels of glycolytic and PPP intermediates. The error bar indicates ±SEM. n = 3. (*) P < 0.05; (**) P < 0.01; (***) P < 0.0001, _t_-test. (D) The schematic of carbon atom transitions and tracers shows that glutamine contributes three carbons for de novo pyrimidine synthesis. (E) The [U13C5]-Gln tracer study shows increased glutamine flux to aspartate and uridine phosphates (M+3) in _Atg7_-deficient tumor cells compared with wild type in HBSS (4 h). (F) Model depicting how autophagy sustains energy charge and nucleotide pools to enable the survival of _Kras_-driven tumor cells in starvation. Glutamine and nucleoside supplementation (green) is sufficient to maintain energy and nucleotide pools to prevent the death of _Atg7_−/− TDCLs in starvation.
Similar articles
- Autophagy is required for mitochondrial function, lipid metabolism, growth, and fate of KRAS(G12D)-driven lung tumors.
Guo JY, White E. Guo JY, et al. Autophagy. 2013 Oct;9(10):1636-8. doi: 10.4161/auto.26123. Epub 2013 Aug 15. Autophagy. 2013. PMID: 23959381 Free PMC article. Review. - Autophagy modulates lipid metabolism to maintain metabolic flexibility for _Lkb1_-deficient _Kras_-driven lung tumorigenesis.
Bhatt V, Khayati K, Hu ZS, Lee A, Kamran W, Su X, Guo JY. Bhatt V, et al. Genes Dev. 2019 Feb 1;33(3-4):150-165. doi: 10.1101/gad.320481.118. Epub 2019 Jan 28. Genes Dev. 2019. PMID: 30692209 Free PMC article. - Autophagy promotes BrafV600E-driven lung tumorigenesis by preserving mitochondrial metabolism.
Strohecker AM, White E. Strohecker AM, et al. Autophagy. 2014 Feb;10(2):384-5. doi: 10.4161/auto.27320. Epub 2013 Dec 17. Autophagy. 2014. PMID: 24362353 Free PMC article. - Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis.
Guo JY, Karsli-Uzunbas G, Mathew R, Aisner SC, Kamphorst JJ, Strohecker AM, Chen G, Price S, Lu W, Teng X, Snyder E, Santanam U, Dipaola RS, Jacks T, Rabinowitz JD, White E. Guo JY, et al. Genes Dev. 2013 Jul 1;27(13):1447-61. doi: 10.1101/gad.219642.113. Genes Dev. 2013. PMID: 23824538 Free PMC article. - Autophagy, Metabolism, and Cancer.
Guo JY, White E. Guo JY, et al. Cold Spring Harb Symp Quant Biol. 2016;81:73-78. doi: 10.1101/sqb.2016.81.030981. Epub 2017 Feb 16. Cold Spring Harb Symp Quant Biol. 2016. PMID: 28209717 Free PMC article. Review.
Cited by
- Autophagy promotes cell survival by maintaining NAD levels.
Kataura T, Sedlackova L, Otten EG, Kumari R, Shapira D, Scialo F, Stefanatos R, Ishikawa KI, Kelly G, Seranova E, Sun C, Maetzel D, Kenneth N, Trushin S, Zhang T, Trushina E, Bascom CC, Tasseff R, Isfort RJ, Oblong JE, Miwa S, Lazarou M, Jaenisch R, Imoto M, Saiki S, Papamichos-Chronakis M, Manjithaya R, Maddocks ODK, Sanz A, Sarkar S, Korolchuk VI. Kataura T, et al. Dev Cell. 2022 Nov 21;57(22):2584-2598.e11. doi: 10.1016/j.devcel.2022.10.008. Dev Cell. 2022. PMID: 36413951 Free PMC article. - Hydrogen-rich water exerts anti-tumor effects comparable to 5-fluorouracil in a colorectal cancer xenograft model.
Asgharzadeh F, Tarnava A, Mostafapour A, Khazaei M, LeBaron TW. Asgharzadeh F, et al. World J Gastrointest Oncol. 2022 Jan 15;14(1):242-252. doi: 10.4251/wjgo.v14.i1.242. World J Gastrointest Oncol. 2022. PMID: 35116114 Free PMC article. - Nucleotide imbalance decouples cell growth from cell proliferation.
Diehl FF, Miettinen TP, Elbashir R, Nabel CS, Darnell AM, Do BT, Manalis SR, Lewis CA, Vander Heiden MG. Diehl FF, et al. Nat Cell Biol. 2022 Aug;24(8):1252-1264. doi: 10.1038/s41556-022-00965-1. Epub 2022 Aug 4. Nat Cell Biol. 2022. PMID: 35927450 Free PMC article. - Targeting drug-tolerant cells: A promising strategy for overcoming acquired drug resistance in cancer cells.
Song X, Lan Y, Zheng X, Zhu Q, Liao X, Liu K, Zhang W, Peng Q, Zhu Y, Zhao L, Chen X, Shu Y, Yang K, Hu J. Song X, et al. MedComm (2020). 2023 Aug 24;4(5):e342. doi: 10.1002/mco2.342. eCollection 2023 Oct. MedComm (2020). 2023. PMID: 37638338 Free PMC article. Review. - Oncogenic KRAS Induces NIX-Mediated Mitophagy to Promote Pancreatic Cancer.
Humpton TJ, Alagesan B, DeNicola GM, Lu D, Yordanov GN, Leonhardt CS, Yao MA, Alagesan P, Zaatari MN, Park Y, Skepper JN, Macleod KF, Perez-Mancera PA, Murphy MP, Evan GI, Vousden KH, Tuveson DA. Humpton TJ, et al. Cancer Discov. 2019 Sep;9(9):1268-1287. doi: 10.1158/2159-8290.CD-18-1409. Epub 2019 Jul 1. Cancer Discov. 2019. PMID: 31263025 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA072720/CA/NCI NIH HHS/United States
- R01 CA193970/CA/NCI NIH HHS/United States
- K22 CA190521/CA/NCI NIH HHS/United States
- R01 CA163591/CA/NCI NIH HHS/United States
- R01 CA130893/CA/NCI NIH HHS/United States
- R01 CA188096/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous