Zika virus infection damages the testes in mice - PubMed (original) (raw)
. 2016 Dec 15;540(7633):438-442.
doi: 10.1038/nature20556. Epub 2016 Oct 31.
Prabagaran Esakky 2, Suzanne M Scheaffer 2, Estefania Fernandez 3, Andrea Drury 2, Derek J Platt 4, Matthew J Gorman 3, Justin M Richner 1, Elizabeth A Caine 1, Vanessa Salazar 1, Kelle H Moley 2 5, Michael S Diamond 1 3 4 6
Affiliations
- PMID: 27798603
- PMCID: PMC5432198
- DOI: 10.1038/nature20556
Zika virus infection damages the testes in mice
Jennifer Govero et al. Nature. 2016.
Abstract
Infection of pregnant women with Zika virus (ZIKV) can cause congenital malformations including microcephaly, which has focused global attention on this emerging pathogen. In addition to transmission by mosquitoes, ZIKV can be detected in the seminal fluid of affected males for extended periods of time and transmitted sexually. Here, using a mouse-adapted African ZIKV strain (Dakar 41519), we evaluated the consequences of infection in the male reproductive tract of mice. We observed persistence of ZIKV, but not the closely related dengue virus (DENV), in the testis and epididymis of male mice, and this was associated with tissue injury that caused diminished testosterone and inhibin B levels and oligospermia. ZIKV preferentially infected spermatogonia, primary spermatocytes and Sertoli cells in the testis, resulting in cell death and destruction of the seminiferous tubules. Less damage was caused by a contemporary Asian ZIKV strain (H/PF/2013), in part because this virus replicates less efficiently in mice. The extent to which these observations in mice translate to humans remains unclear, but longitudinal studies of sperm function and viability in ZIKV-infected humans seem warranted.
Figures
Extended Data Figure 1. ZIKV infection of mature sperm
Mature sperm was harvested from the caudal epididymis of uninfected (left) or ZIKV-infected (day 7, right) mice and processed by ISH with a ZIKV-specific probe. Staining for viral RNA is seen in the ZIKV-infected samples at the head (inset, red arrow) and in the cytoplasmic droplets (inset, green arrows) in the sperm flagellum. Scale bar = 50 μm. Staining was quantitated by microscopy: (i) Uninfected: 81 sperm counted, 0 positive for staining in head, 0 positive for staining in tail; (ii) ZIKV-infected: 93 sperm counted, 25 (27%) positive for staining in head, 57 (61%) positive for staining in tail.
Extended Data Figure 2. Temporal loss of cellularity in the testis after ZIKV infection
Seven week-old WT C57BL/6 mice were treated with 0.5 mg of anti-Ifnar1 at day -1 prior to subcutaneous inoculation of mouse-adapted ZIKV Dakar. Immunohistochemical analysis was performed on testis tissues collected from uninfected (top panels) or ZIKV-infected animals (days 7, 14 or 21 after infection; bottom panels) at 20× (left two images) and 40× (right image) magnification. Staining was performed with antibodies against 3β-HSD (Leydig cells, left panels), TRA98 (germ cells, middle panels), and Lin28a (type A undifferentiated and type B spermatogonia, right panels). Blue arrows indicate staining of Leydig cells (left panels), germ cells (middle panels), and spermatogonial stem cells (right panels). Red arrows indicate areas of virus-induced damage and loss of tissue integrity and specific cellularity. Scale bars = 200, 200, and 50 μm for the grouping of the three sets of images.
Extended Data Figure 3. Histology of the testes at day 28 after infection with ZIKV H/PF/2013
Seven week-old WT C57BL/6 mice were treated with PBS or anti-Ifnar1 at day -1 prior to subcutaneous inoculation in the footpad with 103 FFU of ZIKV H/PF/2013 or 106 FFU of DENV-2. Testes were collected at day 14 (a) or 28 (b) after infection and analyzed for viral RNA by qRT-PCR. Results are pooled from two independent biological experiments and each symbol represents data from an individual mouse. Bars indicate mean values. c. Histological analysis of PFA-fixed testis (left panels) and epididymis (right panels) tissues collected from uninfected or ZIKV-infected animals at day 28 at 20× (left) and 40× (right) magnification. Arrows indicate loss of germ cells and vacuoles in the testis (red), involution of epididymal lumens (yellow) with a mass of residual sperm (blue) and thickened epithelium (green). The images are representative of several independent experiments. Scale bars are indicated in the bottom right corner of the panels. Scale bars = 200 μm.
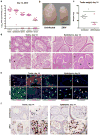
Extended Data Figure 4. ZIKV infection of the testis and epididymis at day 7 in _Axl_-/- and _Rag1_-/- mice
Seven week-old WT, _Axl_-/-, or _Rag1_-/- C57BL/6 mice were treated with 0.5 mg of anti-Ifnar1 at day -1 prior to subcutaneous inoculation in the footpad with 106 FFU of mouse-adapted ZIKV Dakar. a. The indicated tissues were collected at day 7 after infection and analyzed for viral RNA by qRT-PCR. Dashed lines indicate limit of detection of the assays. b. ISH of testis from uninfected or ZIKV-infected WT and _Axl_-/- mice at day 7 with a ZIKV-specific probe. Dark blue arrows indicate Sertoli cells. (Inset) In sections from infected WT and _Axl_-/-mice, the cytoplasm of Sertoli cells is positive for ZIKV RNA (dark brown) with signal absent from prominent nuclei and nucleoli. Scale bar = 50 μm. c. Histology (H & E, left two panels) and ISH (right two panels) of testis from age-matched uninfected or ZIKV-infected (day 13) _Rag1_-/- mice at 20× (left) and 40× (right) magnification for each pair. Scale bars = 200 and 50 μm. In H & E stained testis sections arrows indicate loss of germ cells, and presence of multi-nucleated giant and necrotic cells from ZIKV-infected _Rag1_-/- mice. In ISH, red and blue arrows indicate distribution of ZIKV RNA and Sertoli cells, respectively. d. IF (three left and two right panels) and IHC (three middle) staining of uninfected or ZIKV-infected (day 13) testis and epididymis from _Rag1_-/- mice with antibodies to CD45, TRA98, ETV5, GATA4, Lin28a, 3b-HSD, F4/80 as described in Figure 1 and Extended Data Figure 2. Colored arrows indicate staining for leukocytes (CD45, white), germ cells (TRA98, orange), Sertoli cells (GATA4, magenta), BTB (ETV5, green), type A undifferentiated and type B spermatogonia (Lin28a, black), and Leydig cells (3β-HSD, black) in respective panels. In the IHC staining panels with TRA98, red arrows indicate dying or dead germ cells and tubules without germ cells. White lines demarcate tubules in the seminiferous epithelium. Scale bars = 200 μm for IHC and 50 μm for IF. The images are representative from several different animals.
Extended Data Figure 5. ZIKV infection of the testis and epididymis at ∼day 42. a–b
Seven week-old WT C57BL/6 mice were treated with anti-Ifnar1 at day -1 prior to subcutaneous inoculation in the footpad with 105 FFU of mouse-adapted ZIKV Dakar. a. Testis (left panels) and epididymis (right panels) were collected at day 41 after infection or from age-matched uninfected mice, fixed with PFA, sectioned, stained with H & E, and imaged at 20× (left) and 40× (right) magnification. Arrows show epididymal lumen void of sperm. The images are representative of sections from several independent animals. Scale bars are indicated in the bottom right corner of the panels. Scale bars = 200 and 50 μm. b. The indicated tissues and cells were collected at ∼day 42 after infection (days 41 (n = 3), 42 (n = 4), 43 (n = 3), and 48 (n =1)) and analyzed for viral RNA by qRT-PCR. Dashed line indicates the limit of detection of the assay. Results are pooled from two to three independent biological experiments and each symbol represents data from an individual mouse. Bars indicate median values.
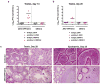
Figure 1. ZIKV infection of the testis and epididymis at day 7. a–c
Seven week-old WT mice were treated with an isotype control (a) or anti-Ifnar1 mAb (2 mg (a) or 0.5 mg (b–c)) at day -1 prior to subcutaneous inoculation with 103 FFU of ZIKV H/PF/2013 (a), 106 FFU of DENV-2 (a), or mouse-adapted ZIKV Dakar (b–c). Tissues and cells were collected at day 7 after infection and analyzed for viral RNA by qRT-PCR (a–b) or infectious virus by plaque assay (c). Dashed lines indicate limit of detection. Results are pooled from two to three independent experiments and each symbol represents data from an individual mouse. Bars indicate median values. Viral RNA was normalized to a standard curve from RNA isolated from infectious virus. d Representative image of testis from an uninfected and ZIKV Dakar-infected mouse at day 7; scale bar = 2 mm. e. Weight of testis from uninfected and ZIKV-infected mice at day 7. Results are pooled from two independent experiments. Mean values were not statistically different (n.s.; unpaired t test). f-h. Histological, immunohistochemical, and ISH analysis of testis (left panels) and epididymis (right panels) collected from uninfected or ZIKV-infected animals at 20× (left) and 40× (right) magnification. f. Hematoxylin and eosin (H & E) staining. g. Immunofluorescence (IF) staining of uninfected or ZIKV-infected testis and epididymis tissue sections with antibodies to CD45 (pan-leukocyte), TRA98 (germ cells), ETV5 (BTB), GATA4 (Sertoli cells), and F4/80 (macrophages). Arrows indicate staining for leukocytes (white), germ cells (orange), Sertoli cells (magenta), and BTB (green). White lines demarcate tubules of seminiferous epithelium. h. ISH with a ZIKV-specific probe. Arrows indicate cells positive for ZIKV RNA (spermatogonia and primary spermatocytes (red), Sertoli cells (green), and epididymis lumenal sperm (blue)). The images in panels f-h are representative of several independent experiments. Scale bars = 200 and 50 μm for H & E and ISH and 50 μm for IF.
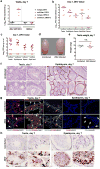
Figure 2. ZIKV infection of the testis and epididymis at day 14. a–b
Seven week-old WT mice were treated with 0.5 mg of anti-Ifnar1 at day -1 prior to subcutaneous inoculation of mouse-adapted ZIKV Dakar. Tissues and cells were collected at day 14 and analyzed for viral RNA by qRT-PCR (a). Dashed lines indicate limit of detection. Results are pooled from three independent experiments. Bars indicate median values. b. Representative images of testis from an uninfected and ZIKV-infected mouse at day 14; scale bar = 2 mm. c. Weight of testis from uninfected and ZIKV-infected mice at day 14. Results are pooled from two independent experiments (*, P < 0.05, Mann-Whitney test). d–f. Histological, immunohistochemical, and ISH analysis of testis (left panels) and epididymis (right panels) tissues collected from uninfected or ZIKV-infected animals at 20× (left) and 40× (right) magnification. d. H & E staining. Arrows denote involution of seminiferous tubules in the testis (red), constricted epididymal lumens (yellow) with a mass of residual sperm (blue) and thickened epithelium (green). e. IF staining of uninfected or ZIKV-infected testes and epididymis tissues as described in Figure 1. Arrows indicate staining for leukocytes (white), germ cells (orange), Sertoli cells (magenta), BTB (green), and macrophages (cyan). White lines demarcate tubules in the seminiferous epithelium. f. ISH. Arrows indicate cells positive for ZIKV RNA (testicular cells (red) and epididymis lumenal sperm and cilia on the inner layer of epididymal epithelium (blue)). The images in panels d–f are representative of several independent experiments. Scale bars = 200 and 50 μm for H & E and ISH and 50 μm for IF.
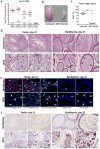
Figure 3. ZIKV infection of the testis and epididymis at day 21
a Seven week-old WT mice were treated with 0.5 mg of anti-Ifnar1 at day -1 prior to subcutaneous inoculation of mouse-adapted ZIKV Dakar. Tissues and cells were collected at day 21 after infection and analyzed for viral RNA by qRT-PCR. Dashed lines indicate limit of detection. Results are pooled from two independent experiments. Bars indicate median values. b. Representative images of testis from an uninfected and ZIKV-infected mouse at day 21; scale bar = 2 mm. c. Weight of testis from uninfected and ZIKV-infected mice at day 21. Results are pooled from two independent experiments (*, P < 0.05, Mann-Whitney test). d. Histological analysis of testis (left panels) and epididymis (right panels) tissues collected from uninfected or ZIKV-infected animals at 20× (left) and 40× (right) magnification and stained with H & E. Arrows indicate involution of seminiferous tubules in the testis (red), shrunken epididymal lumens (yellow) with a mass of residual sperm (blue). e. IF staining of uninfected or ZIKV-infected testis and epididymis tissues as described in Figure 1 and 2. Arrows indicate staining for leukocytes (white), germ cells (orange), Sertoli cells (magenta), BTB (green), and macrophages (cyan). White lines demarcate tubules in the seminiferous epithelium f. ISH. Arrows indicate cells positive for ZIKV RNA (testicular cells (red) and epididymal lumenal sperm (blue)). The images in panels d-f are representative of several independent experiments. Scale bars = 200 and 50 μm for H & E and ISH and 50 μm for IF.
Figure 4. Consequences of ZIKV infection of the testis and epididymis
a Testosterone (left) and inhibin B (right) levels of testis homogenates from uninfected (UNINF) and ZIKV-infected (days 7, 14, or 21) mice. b–c. Computer-assisted sperm analysis (total (left panels) and motile (right panels)) on samples obtained from the caudal epididymis of ZIKV-infected males immediately after euthanasia at days (b) 14 or (c) ∼42 (range of 41 to 48 days) after infection or age-matched uninfected males. a–c. Results are pooled from two to five independent experiments. Bars indicate median values, and differences between uninfected and ZIKV-infected animals were evaluated (a, *, P < 0.05; **, P < 0.01; ***, P < 0.001; ANOVA (Kruskal-Wallis) with a multiple comparison correction; b–c, **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; Mann-Whitney test. Dashed lines indicate the limit of sensitivity of the assay. d. Fertility studies. Age-matched uninfected or ZIKV-infected males (at days 7, 16 or 26 after infection (n = 4 to 5 male mice for each time point)) were mated with individual 8 week-old female WT mice (n = 4-5 female per round with 3 independent rounds performed) for five days and then separated. Ten days later, we evaluated the pregnancy rate (left, symbols correspond to the percentage of 5 females becoming pregnant for a given trial) and the total number of viable or resorbed fetuses for each round (right) (*, P < 0.05; **, P < 0.01; unpaired Student's t test). e. TUNEL staining of testis (left panels) and epididymis (right panels) from uninfected or ZIKV-infected mice at days 7, 14, or 21. TUNEL staining in germ and somatic cells of the testis and lumenal sperm in the epididymis is shown (green staining and white arrows). The images are representative of several independent experiments. Scale bars = 100 μm.
Comment in
- Disease watch: Zika virus - concerns for male fertility.
Holmes D. Holmes D. Nat Rev Endocrinol. 2017 Jan;13(1):3. doi: 10.1038/nrendo.2016.191. Epub 2016 Nov 18. Nat Rev Endocrinol. 2017. PMID: 27857129 No abstract available.
Similar articles
- Sertoli Cells Are Susceptible to ZIKV Infection in Mouse Testis.
Sheng ZY, Gao N, Wang ZY, Cui XY, Zhou DS, Fan DY, Chen H, Wang PG, An J. Sheng ZY, et al. Front Cell Infect Microbiol. 2017 Jun 21;7:272. doi: 10.3389/fcimb.2017.00272. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28680856 Free PMC article. - Routes of Zika virus dissemination in the testis and epididymis of immunodeficient mice.
Tsetsarkin KA, Maximova OA, Liu G, Kenney H, Teterina N, Bloom ME, Grabowski JM, Mlera L, Nagata BM, Moore I, Martens C, Amaro-Carambot E, Lamirande EW, Whitehead SS, Pletnev AG. Tsetsarkin KA, et al. Nat Commun. 2018 Dec 17;9(1):5350. doi: 10.1038/s41467-018-07782-x. Nat Commun. 2018. PMID: 30559387 Free PMC article. - Zika Virus Infects Human Sertoli Cells and Modulates the Integrity of the In Vitro Blood-Testis Barrier Model.
Siemann DN, Strange DP, Maharaj PN, Shi PY, Verma S. Siemann DN, et al. J Virol. 2017 Oct 27;91(22):e00623-17. doi: 10.1128/JVI.00623-17. Print 2017 Nov 15. J Virol. 2017. PMID: 28878076 Free PMC article. - The Cellular Impact of the ZIKA Virus on Male Reproductive Tract Immunology and Physiology.
Almeida RDN, Braz-de-Melo HA, Santos IO, Corrêa R, Kobinger GP, Magalhaes KG. Almeida RDN, et al. Cells. 2020 Apr 18;9(4):1006. doi: 10.3390/cells9041006. Cells. 2020. PMID: 32325652 Free PMC article. Review. - Zika virus - an overview.
Zanluca C, Dos Santos CN. Zanluca C, et al. Microbes Infect. 2016 May;18(5):295-301. doi: 10.1016/j.micinf.2016.03.003. Epub 2016 Mar 16. Microbes Infect. 2016. PMID: 26993028 Review.
Cited by
- Zika virus tropism during early infection of the testicular interstitium and its role in viral pathogenesis in the testes.
Tsetsarkin KA, Acklin JA, Liu G, Kenney H, Teterina NL, Pletnev AG, Lim JK. Tsetsarkin KA, et al. PLoS Pathog. 2020 Jul 2;16(7):e1008601. doi: 10.1371/journal.ppat.1008601. eCollection 2020 Jul. PLoS Pathog. 2020. PMID: 32614902 Free PMC article. - Sexual Transmission of Arboviruses: A Systematic Review.
Blitvich BJ, Magalhaes T, Laredo-Tiscareño SV, Foy BD. Blitvich BJ, et al. Viruses. 2020 Aug 25;12(9):933. doi: 10.3390/v12090933. Viruses. 2020. PMID: 32854298 Free PMC article. - Live Zika virus chimeric vaccine candidate based on a yellow fever 17-D attenuated backbone.
Touret F, Gilles M, Klitting R, Aubry F, de Lamballerie X, Nougairède A. Touret F, et al. Emerg Microbes Infect. 2018 Sep 26;7(1):161. doi: 10.1038/s41426-018-0161-7. Emerg Microbes Infect. 2018. PMID: 30254297 Free PMC article. - Are the Organoid Models an Invaluable Contribution to ZIKA Virus Research?
Marrazzo P, Cricca M, Nastasi C. Marrazzo P, et al. Pathogens. 2021 Sep 24;10(10):1233. doi: 10.3390/pathogens10101233. Pathogens. 2021. PMID: 34684182 Free PMC article. Review. - A gossypol derivative effectively protects against Zika and dengue virus infection without toxicity.
Gao Y, Tai W, Wang X, Jiang S, Debnath AK, Du L, Chen S. Gao Y, et al. BMC Biol. 2022 Jun 15;20(1):143. doi: 10.1186/s12915-022-01344-w. BMC Biol. 2022. PMID: 35706035 Free PMC article.
References
Additional References (Methods)
Publication types
MeSH terms
Substances
Grants and funding
- U19 AI083019/AI/NIAID NIH HHS/United States
- R01 AI073755/AI/NIAID NIH HHS/United States
- R01 HD083895/HD/NICHD NIH HHS/United States
- P41 GM103422/GM/NIGMS NIH HHS/United States
- P50 HD028934/HD/NICHD NIH HHS/United States
- T32 AI007163/AI/NIAID NIH HHS/United States
- T32 AI007172/AI/NIAID NIH HHS/United States
- R01 AI104972/AI/NIAID NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- P41 GM103422-35/GM/NIGMS NIH HHS/United States
- R01 AI101400/AI/NIAID NIH HHS/United States
- R01 HD065435/HD/NICHD NIH HHS/United States