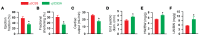Hemoglobin S-nitrosylation plays an essential role in cardioprotection - PubMed (original) (raw)
Hemoglobin S-nitrosylation plays an essential role in cardioprotection
Rongli Zhang et al. J Clin Invest. 2016.
Abstract
Homeostatic control of tissue oxygenation is achieved largely through changes in blood flow that are regulated by the classic physiological response of hypoxic vasodilation. The role of nitric oxide (NO) in the control of blood flow is a central tenet of cardiovascular biology. However, extensive evidence now indicates that hypoxic vasodilation entails S-nitrosothiol-based (SNO-based) vasoactivity (rather than NO per se) and that this activity is conveyed substantially by the βCys93 residue in hemoglobin. Thus, tissue oxygenation in the respiratory cycle is dependent on S-nitrosohemoglobin. This perspective predicts that red blood cells (RBCs) may play an important but previously undescribed role in cardioprotection. Here, we have found that cardiac injury and mortality in models of myocardial infarction and heart failure were greatly enhanced in mice lacking βCys93 S-nitrosylation. In addition, βCys93 mutant mice exhibited adaptive collateralization of cardiac vasculature that mitigated ischemic injury and predicted outcomes after myocardial infarction. Enhanced myopathic injury and mortality across different etiologies in the absence of βCys93 confirm the central cardiovascular role of RBC-derived SNO-based vasoactivity and point to a potential locus of therapeutic intervention. Our findings also suggest the possibility that RBCs may play a previously unappreciated role in heart disease.
Conflict of interest statement
J.S. Stamler has financial interests in Nivalis Therapeutics Inc., Adamas Pharmaceuticals, and LifeHealth. J.D. Reynolds has a financial interest in Miach Medical Innovations. J.S. Stamler and J.D. Reynolds have licensed technology to Novartis.
Figures
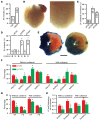
Figure 1. Enhanced MI-induced cardiac injury and mortality in the absence of βCys93.
(A) IR-induced mortality is greatly enhanced in mice lacking βCys93 (C93A = γβC93A and βC93A) versus control mice (C93 = γβC93). In panels A, C, and D, numbers within histograms specify absolute ratios for each observation (B) Representative angiography illustrating collateralized circulation of the LV free wall to supply the apex in a mutant βC93A mouse. Lower (left; ×6.7) and higher (right; ×15.8) magnification views are shown. (C) Collateralization supplying the LV is significantly more likely in γβC93A and βC93A versus γβC93 mice. *P < 0.0001 for γβC93 versus γβC93A and **P = 0.0043 for γβC93 versus βC93A, respectively, by Fisher’s exact test (2-sided). The difference in collateralization between γβC93A and βC93A mice was not significant (_P_ > 0.05). (D) Mortality during 24 hours of IR is determined by the presence versus absence of collateral circulatory supply of the LV and is enhanced in γβC93A and βC93A versus γβC93 mice. *P < 0.0001 vs. collateral by Fisher’s exact test (2-sided). (E) Representative Evans blue/TTC-stained LV slices illustrating areas of recovery (red) and necrosis (white) in a γβC93 mouse (left) and in a γβC93A mouse (right). Scale bar: 2 mm. (F) In γβC93A versus γβC93 control mice surviving 24 hours after IR, the area of recovery (Rec/LV; LV, total LV area) is significantly diminished and the area of necrosis (AON/LV) and the ratio of area of necrosis/area at risk (AON/AAR) are significantly enhanced, and enhanced injury was evident in the absence of βC93 even when collateralization was present. (G) Echocardiography of surviving mice reveals that LV ejection fraction (EF) and fractional shortening (FS) are significantly diminished in γβC93A versus γβC93 control mice, and these differences were evident even when collateralization was present. (H) Thickening of the LV anterior and posterior walls in the absence of collateral blood supply is significantly diminished in γβC93A versus γβC93 mice. In F–H, n = 3–7 for mice with collateral blood supply and n = 3–9 for mice without collateral blood supply. *P < 0.05 vs. γβC93 by Student’s t test (2-tailed).
Figure 2. Assessment of acute effects of pressure overload (TAC) on cardiac function in C93A mutant animals.
γβC93 and γβC93A mice were compared at 2 days. (A–C) Echocardiography revealed significantly decreased ejection fraction (A), fractional shortening (B), and cardiac output (C) in γβC93A versus γβC93 mice. (D) End systolic diameter (end systolic diam.) was significantly greater in γβC93A versus γβC93 mice. (E and F) The ratios of heart weight with respect to body weight (HW/BW) (E) and lung weight with respect to body weight (LW/BW) (F) were significantly increased in γβC93A versus γβC93 mice. n = 5–7. *P < 0.05 vs. γβC93 by Student’s t test (2-tailed).
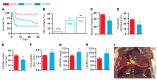
Figure 3. Assessment of chronic effects of pressure overload (TAC) on mortality and injury in C93A mutant animals.
Comparisons among γβC93, βC93A, and γβC93A mice were made following 4 weeks of TAC. (A) A Kaplan-Meier curve illustrates that, within 5 days after TAC, only 1 of 18 γβC93 control animals died (5.6% mortality), whereas 7 of 11 γβC93A mice died (63.6% mortality) before the population stabilized. Also, 12 of 23 βC93A mice died (52.2% mortality), with deaths accumulating over the full TAC time course. (B) Cumulative mortality over 4 weeks was significantly greater for βC93A and γβC93A versus γβC93 mice and did not differ significantly between βC93A and γβC93A mice. *P = 0.0019 vs. γβC93 for γβC93A and **P = 0.0014 vs. γβC93 for γβC93A by Fisher’s exact test (2-sided). (C–F) Echocardiography in mice surviving the 4-week TAC interval revealed greater impairment of LV function in βC93A versus γβC93 mice with respect to ejection fraction (C), fractional shortening (D), cardiac output (E), and end systolic diameter (LV dilation) (F). (G and H) Ratios of heart weight with respect to body weight (G) and lung weight with respect to body weight (H) were significantly increased in βC93A versus γβC93 mice, indicative of increased myocardial hypertrophy and pulmonary edema, respectively. In C–H, n = 11 (γβC93) or 12 (βC93A); P < 0.01 by Student’s t test (2-tailed). (I) Post-mortem examination revealed lung edema and pleural effusion (yellow arrows; a representative βC93A mouse is shown).
Comment in
- Cardioprotective role of S-nitrosylated hemoglobin from rbc.
Piantadosi CA. Piantadosi CA. J Clin Invest. 2016 Dec 1;126(12):4402-4403. doi: 10.1172/JCI91303. Epub 2016 Nov 14. J Clin Invest. 2016. PMID: 27841761 Free PMC article.
Similar articles
- Red Blood Cell-Mediated S-Nitrosohemoglobin-Dependent Vasodilation: Lessons Learned from a β-Globin Cys93 Knock-In Mouse.
Premont RT, Reynolds JD, Zhang R, Stamler JS. Premont RT, et al. Antioxid Redox Signal. 2021 Apr 20;34(12):936-961. doi: 10.1089/ars.2020.8153. Epub 2020 Jul 23. Antioxid Redox Signal. 2021. PMID: 32597195 Free PMC article. Review. - Cardioprotective role of S-nitrosylated hemoglobin from rbc.
Piantadosi CA. Piantadosi CA. J Clin Invest. 2016 Dec 1;126(12):4402-4403. doi: 10.1172/JCI91303. Epub 2016 Nov 14. J Clin Invest. 2016. PMID: 27841761 Free PMC article. - Essential Role of Hemoglobin βCys93 in Cardiovascular Physiology.
Premont RT, Stamler JS. Premont RT, et al. Physiology (Bethesda). 2020 Jul 1;35(4):234-243. doi: 10.1152/physiol.00040.2019. Physiology (Bethesda). 2020. PMID: 32490751 Free PMC article. Review. - Optimized S-nitrosohemoglobin Synthesis in Red Blood Cells to Preserve Hypoxic Vasodilation Via _β_Cys93.
Hausladen A, Qian Z, Zhang R, Premont RT, Stamler JS. Hausladen A, et al. J Pharmacol Exp Ther. 2022 Jul;382(1):1-10. doi: 10.1124/jpet.122.001194. Epub 2022 May 5. J Pharmacol Exp Ther. 2022. PMID: 35512801 Free PMC article. - SNO-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation.
Isbell TS, Sun CW, Wu LC, Teng X, Vitturi DA, Branch BG, Kevil CG, Peng N, Wyss JM, Ambalavanan N, Schwiebert L, Ren J, Pawlik KM, Renfrow MB, Patel RP, Townes TM. Isbell TS, et al. Nat Med. 2008 Jul;14(7):773-7. doi: 10.1038/nm1771. Epub 2008 May 30. Nat Med. 2008. PMID: 18516054 Free PMC article.
Cited by
- Nitric oxide loading reduces sickle red cell adhesion and vaso-occlusion in vivo.
McMahon TJ, Shan S, Riccio DA, Batchvarova M, Zhu H, Telen MJ, Zennadi R. McMahon TJ, et al. Blood Adv. 2019 Sep 10;3(17):2586-2597. doi: 10.1182/bloodadvances.2019031633. Blood Adv. 2019. PMID: 31484636 Free PMC article. - Role of Nitric Oxide Carried by Hemoglobin in Cardiovascular Physiology: Developments on a Three-Gas Respiratory Cycle.
Premont RT, Reynolds JD, Zhang R, Stamler JS. Premont RT, et al. Circ Res. 2020 Jan 3;126(1):129-158. doi: 10.1161/CIRCRESAHA.119.315626. Epub 2019 Oct 8. Circ Res. 2020. PMID: 31590598 Free PMC article. Review. - Integrated Dissection of Cysteine Oxidative Post-translational Modification Proteome During Cardiac Hypertrophy.
Wang J, Choi H, Chung NC, Cao Q, Ng DCM, Mirza B, Scruggs SB, Wang D, Garlid AO, Ping P. Wang J, et al. J Proteome Res. 2018 Dec 7;17(12):4243-4257. doi: 10.1021/acs.jproteome.8b00372. Epub 2018 Aug 31. J Proteome Res. 2018. PMID: 30141336 Free PMC article. - Inhaled nitric oxide: role in the pathophysiology of cardio-cerebrovascular and respiratory diseases.
Signori D, Magliocca A, Hayashida K, Graw JA, Malhotra R, Bellani G, Berra L, Rezoagli E. Signori D, et al. Intensive Care Med Exp. 2022 Jun 27;10(1):28. doi: 10.1186/s40635-022-00455-6. Intensive Care Med Exp. 2022. PMID: 35754072 Free PMC article. Review. - S-Nitrosohemoglobin Levels and Patient Outcome After Transfusion During Pediatric Bypass Surgery.
Matto F, Kouretas PC, Smith R, Ostrowsky J, Cina AJ, Hess DT, Stamler JS, Reynolds JD. Matto F, et al. Clin Transl Sci. 2018 Mar;11(2):237-243. doi: 10.1111/cts.12530. Epub 2017 Dec 12. Clin Transl Sci. 2018. PMID: 29232772 Free PMC article.
References
- Ross JM, Fairchild HM, Weldy J, Guyton AC. Autoregulation of blood flow by oxygen lack. Am J Physiol. 1962;202:21–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases