ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways - PubMed (original) (raw)
ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways
Teneema Kuriakose et al. Sci Immunol. 2016.
Abstract
The interferon-inducible protein Z-DNA binding protein 1 (ZBP1, also known as DNA-dependent activator of IFN-regulatory factors (DAI) and DLM-1) was identified as a dsDNA sensor, which instigates innate immune responses. However, this classification has been disputed and whether ZBP1 functions as a pathogen sensor during an infection has remained unknown. Herein, we demonstrated ZBP1-mediated sensing of the influenza A virus (IAV) proteins NP and PB1, triggering cell death and inflammatory responses via the RIPK1-RIPK3-Caspase-8 axis. ZBP1 regulates NLRP3 inflammasome activation as well as induction of apoptosis, necroptosis and pyroptosis in IAV-infected cells. Importantly, ZBP1 deficiency protected mice from mortality during IAV infection owing to reduced inflammatory responses and epithelial damage. Overall, these findings indicate that ZBP1 is an innate immune sensor of IAV and highlight its importance in the pathogenesis of IAV infection.
Keywords: DAI; IFNs; NLRP3; RIPK3; ZBP1; apoptosis; caspase-1; caspase-8; inflammasome; influenza virus; necroptosis; pyroptosis.
Conflict of interest statement
Competing financial interests: The authors declare no competing financial interests.
Figures
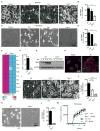
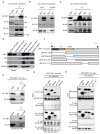
Figure 1. The IFN-inducible protein ZBP1 mediates cell death in response to IAV infection
(A and B) Microscopic analysis and quantification of cell death by LDH release in BMDMs infected with IAV 16 h pi (p= 0.0131; n=4; two-tailed t test). (C and D) Microscopic analysis and quantification of cell death by LDH release in ear fibroblasts infected with IAV 16 h pi (p= 2.92096*10−6; n=4; two-tailed t test). (E) Microarray gene expression dataset enriched for nucleic acid sensing pathways with higher or lower expression in WT and _Ifnar1_−/− BMDMs 9 h pi with IAV (one experiment with two biological replicates per genotype). (F) Real-time quantitative RT-PCR analysis of Zbp1 expression in WT and _Ifnar1_−/− BMDMs 9 h pi with IAV (one experiment with two biological replicates per genotype). (G) Immunoblot analysis of ZBP1 and GAPDH (loading control) in unprimed BMDMs (0–12) h pi with IAV (n=3). (H) Immunostaining for ZBP1 in WT BMDMs infected with IAV for 16 h (n=2). (I and J) Microscopic analysis and quantification of cell death by LDH release in BMDMs infected with IAV 16 h pi (p= 0.0001; n=5; one-way ANOVA). (K and L) Microscopic analysis and quantification of cell death by LDH release in fibroblasts infected with IAV 16 h pi (p= 0.000460657; n=3; two-tailed t test). (M) Real time analysis of the kinetics of cell death in primary ear fibroblasts infected with IAV (MOI 10) (one experiment with three biological replicates per genotype).
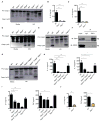
Figure 2. ZBP1 regulates NLRP3 inflammasome activation and proinflammatory cytokine production during IAV infection
(A) Immunoblot analysis of pro-caspase-1 and caspase-1 subunit p20 in BMDMs 16 h pi with IAV (n=4)..(B and C) Levels of IL-1β and IL-18 in cell culture supernatants 16 h pi with IAV; (p= 0.0007 (IL-1β); p = 0.0015 (IL-18); n=3; one-way ANOVA)..(D and E) Immunoblot analysis of pro-caspase-1 and caspase-1 subunit p20 in BMDMs 16 h pi with VSV (Indiana strain) or IAV (n=3)..(F) Immunoprecipitation of ZBP1 from lysates of WT BMDMs infected with IAV for 16 h, and immunoblotted for ZBP1 and RIPK3 (n=3)..(G) Immunoblot analysis of pro-caspase-1 and caspase-1 subunit p20 in BMDMs 16 h pi with IAV (n=3)..(H) Levels of IL-1β and IL-18 in cell culture supernatants 16 h pi with IAV; (p=0.0003 (IL-1β); p=0.0033 (IL-18); n=3; one-way ANOVA)..(I and J) Levels of TNF and IL-6 in cell culture supernatants 16 h pi with IAV;.(I) (p=0.0003 (IL-6; one way ANOVA); p=0.04585299 (TNF; two-tailed t test) n=3);.(J) (p=0.00427068 (IL-6); p=0.01547619 (TNF;); n=3; two-tailed t test).
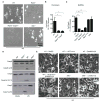
Figure 3. ZBP1 mediates RIPK3-dependent induction of apoptotic and necroptotic cell death pathways during IAV infection
(A and B) Microscopic analysis and quantification of cell death by LDH release in fibroblasts infected with IAV 16 h pi; (p=0.0016; n=3; one-way ANOVA)..(C) Quantification of cell death by LDH release in BMDMs infected with IAV 16 h pi (p=0.0001; n=4; one-way ANOVA)..(D) Immunoblot analysis of the pro- and cleaved-forms of caspase-8, caspase-3 and caspase-7 in BMDMs 16 h pi with IAV (n=3)..(E) Microscopic analysis of BMDMs infected with IAV for 16 h in the absence or presence of inhibitors (n=3).
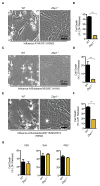
Figure 4. ZBP1 regulates cell death in response to both mouse-adapted and human strains of IAV, but not in response to RNA viruses belonging to Paramyxoviriadae and Rhabdoviridae family
(A to F) Microscopic analysis and quantification of cell death by LDH release in fibroblasts infected with mouse adapted Influenza A/X31 (H3N2), Influenza A/Brisbane/59/2007 (H1N1) and A/Switzerland/9715293/2013 (H3N2) 16h pi (p= 4.5184*10−5 (X31); p=0.02523 (A/Brisbane); p=0.00671454 (A/Switzerland); n=4; two-tailed t test).(G) Quantification of cell death by LDH release in BMDMs infected with VSV (MOI 10), SeV (MOI 8) and RSV (MOI 10) 16 h pi (n=3).
Figure 5. ZBP1 functions as a sensor of IAV infection by interacting with IAV PB1 and NP proteins
(A) Immunoprecipitation of endogenous ZBP1 from lysates of WT BMDMs infected with IAV for 16 h, and immunoblotted for ZBP1, M1, NS1 and HA; (n= 4)..(B) Immunoprecipitation of endogenous ZBP1 from lysates of WT or _Zbp1_−/− fibroblasts infected with IAV for 16 h, and immunoblotted for ZBP1, PB1 and NP; (n= 3)..(C) Immunoprecipitation of ZBP1, PB1 and NP from lysates of WT fibroblasts infected with IAV for 16 h, and immunoblotted for ZBP1, PB1 and NP; (n= 3)..(D) Immunoblot analysis of ZBP1, PB1, NP and GAPDH from whole cell lysates, nuclear and cytoplasmic fractions of WT fibroblasts infected with IAV for 8 h; (n=3)..(E) Immunoprecipitation of HA-tagged ZBP1 from lysates of 239T cells expressing HA-tagged ZBP1 transfected with plasmids expressing PB1 or NP, and immunoblotted for ZBP1, PB1 and NP; (n= 2)..(F) Domain architecture of murine ZBP1 and schematics of the constructs used in this study..(G and H) Immunoprecipitation of HA-tagged ZBP1 from lysates of 239T cells expressing HA-tagged ZBP1 constructs infected with IAV for 16 h, and immunoblotted for the IAV proteins PB1, HA and NP, and HA tag; (n= 2).
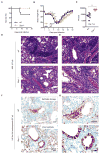
Figure 6. ZBP1 promotes inflammatory responses and epithelial damage during IAV infection in vivo.
(A) Survival analysis of female WT and _Zbp1_−/− mice infected with 1000pfu (approximately one LD50) of IAV; (p=0.0819; n = 15 (WT) and 9 (_Zbp1_−/−) mice; Mantel-Cox test)..(B) Body weight (%) of WT and _Zbp1_−/−mice 0–18 d after infection compared to pre-infection body weight (set as 100%); (p=0.02743813 (D5); 0.00103121 (D6); 0.00081354 (D7); 0.05424982 (D14); 0.0231683 (D15); 0.02152296 (D16); WT (n = 21) and _Zbp1_−/− (n = 15); two-tailed t test)..(C) Lung viral titers in WT and _Zbp1_−/− mice infected with IAV for 7 days; (p=0.0022; n=6; Mann Whitney test). H &E staining (D and E; E is an inset of D) and immunohistochemistry analysis for IAV NP protein (F and G; G is an inset of F) of lungs from WT and _Zbp1_−/− mice infected with IAV for 7 days; (n=6).
Similar articles
- The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis).
Zheng M, Kanneganti TD. Zheng M, et al. Immunol Rev. 2020 Sep;297(1):26-38. doi: 10.1111/imr.12909. Epub 2020 Jul 29. Immunol Rev. 2020. PMID: 32729116 Free PMC article. Review. - IRF1 Is a Transcriptional Regulator of ZBP1 Promoting NLRP3 Inflammasome Activation and Cell Death during Influenza Virus Infection.
Kuriakose T, Zheng M, Neale G, Kanneganti TD. Kuriakose T, et al. J Immunol. 2018 Feb 15;200(4):1489-1495. doi: 10.4049/jimmunol.1701538. Epub 2018 Jan 10. J Immunol. 2018. PMID: 29321274 Free PMC article. - Newly Identified Function of Caspase-6 in ZBP1-mediated Innate Immune Responses, NLRP3 Inflammasome Activation, PANoptosis, and Host Defense.
Zheng M, Kanneganti TD. Zheng M, et al. J Cell Immunol. 2020;2(6):341-347. doi: 10.33696/immunology.2.064. J Cell Immunol. 2020. PMID: 33426542 Free PMC article. - ZBP1/DAI ubiquitination and sensing of influenza vRNPs activate programmed cell death.
Kesavardhana S, Kuriakose T, Guy CS, Samir P, Malireddi RKS, Mishra A, Kanneganti TD. Kesavardhana S, et al. J Exp Med. 2017 Aug 7;214(8):2217-2229. doi: 10.1084/jem.20170550. Epub 2017 Jun 20. J Exp Med. 2017. PMID: 28634194 Free PMC article. - ZBP1: Innate Sensor Regulating Cell Death and Inflammation.
Kuriakose T, Kanneganti TD. Kuriakose T, et al. Trends Immunol. 2018 Feb;39(2):123-134. doi: 10.1016/j.it.2017.11.002. Epub 2017 Nov 25. Trends Immunol. 2018. PMID: 29236673 Free PMC article. Review.
Cited by
- The assembly and activation of the PANoptosome promote porcine granulosa cell programmed cell death during follicular atresia.
Wu H, Han Y, Liu J, Zhao R, Dai S, Guo Y, Li N, Yang F, Zeng S. Wu H, et al. J Anim Sci Biotechnol. 2024 Nov 5;15(1):147. doi: 10.1186/s40104-024-01107-3. J Anim Sci Biotechnol. 2024. PMID: 39497227 Free PMC article. - Mechanism of lactic acidemia-promoted pulmonary endothelial cells death in sepsis: role for CIRP-ZBP1-PANoptosis pathway.
Gong T, Wang QD, Loughran PA, Li YH, Scott MJ, Billiar TR, Liu YT, Fan J. Gong T, et al. Mil Med Res. 2024 Oct 28;11(1):71. doi: 10.1186/s40779-024-00574-z. Mil Med Res. 2024. PMID: 39465383 Free PMC article. - Taking AIM at Influenza: The Role of the AIM2 Inflammasome.
Xu DW, Tate MD. Xu DW, et al. Viruses. 2024 Sep 27;16(10):1535. doi: 10.3390/v16101535. Viruses. 2024. PMID: 39459869 Free PMC article. Review. - Cytoplasmic DNA and AIM2 inflammasome in RA: where they come from and where they go?
Xu C, Jing W, Liu C, Yuan B, Zhang X, Liu L, Zhang F, Chen P, Liu Q, Wang H, Du X. Xu C, et al. Front Immunol. 2024 Oct 10;15:1343325. doi: 10.3389/fimmu.2024.1343325. eCollection 2024. Front Immunol. 2024. PMID: 39450183 Free PMC article. Review.
References
- WHO. Influenza (Seasonal) Fact sheet N0 211. 2009 [updated April 2009; cited 2012 June 12, 2012]. Available from: http://www.who.int/mediacentre/factsheets/fs211/en/index.html.
- Sanders CJ, Vogel P, McClaren JL, Bajracharya R, Doherty PC, Thomas PG. Compromised respiratory function in lethal influenza infection is characterized by the depletion of type I alveolar epithelial cells beyond threshold levels. American journal of physiology Lung cellular and molecular physiology. 2013;304:L481–488. doi: 10.1152/ajplung.00343.2012. published online EpubApr 1. - DOI - PMC - PubMed
Grants and funding
- R01 AI101935/AI/NIAID NIH HHS/United States
- R01 AI124346/AI/NIAID NIH HHS/United States
- P30 CA021765/CA/NCI NIH HHS/United States
- R01 CA163507/CA/NCI NIH HHS/United States
- R37 AI101935/AI/NIAID NIH HHS/United States
- R01 AR056296/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous