Circulating ACE2 activity correlates with cardiovascular disease development - PubMed (original) (raw)
. 2016 Dec 12;17(4):1470320316668435.
doi: 10.1177/1470320316668435. Print 2016 Oct.
Miklós Fagyas 1 2, Attila Kertész 2, Attila Borbély 2, Csaba Jenei 2, Orsolya Bene 2, Zoltán Csanádi 2, Walter J Paulus 3, István Édes 2, Zoltán Papp 1, Attila Tóth 1, Erzsébet Lizanecz 4 2
Affiliations
- PMID: 27965422
- PMCID: PMC5843890
- DOI: 10.1177/1470320316668435
Circulating ACE2 activity correlates with cardiovascular disease development
Katalin Úri et al. J Renin Angiotensin Aldosterone Syst. 2016.
Abstract
It was shown recently that angiotensin-converting enzyme activity is limited by endogenous inhibition in vivo, highlighting the importance of angiotensin II (ACE2) elimination. The potential contribution of the ACE2 to cardiovascular disease progression was addressed. Serum ACE2 activities were measured in different clinical states (healthy, n=45; hypertensive, n=239; heart failure (HF) with reduced ejection fraction (HFrEF) n=141 and HF with preserved ejection fraction (HFpEF) n=47). ACE2 activity was significantly higher in hypertensive patients (24.8±0.8 U/ml) than that in healthy volunteers (16.2±0.8 U/ml, p=0.01). ACE2 activity further increased in HFrEF patients (43.9±2.1 U/ml, p=0.001) but not in HFpEF patients (24.6±1.9 U/ml) when compared with hypertensive patients. Serum ACE2 activity negatively correlated with left ventricular systolic function in HFrEF, but not in hypertensive, HFpEF or healthy populations. Serum ACE2 activity had a fair diagnostic value to differentiate HFpEF from HFrEF patients in this study. Serum ACE2 activity correlates with cardiovascular disease development: it increases when hypertension develops and further increases when the cardiovascular disease further progresses to systolic dysfunction, suggesting that ACE2 metabolism plays a role in these processes. In contrast, serum ACE2 activity does not change when hypertension progresses to HFpEF, suggesting a different pathomechanism for HFpEF, and proposing a biomarker-based identification of these HF forms.
Keywords: Angiotensin-converting enzyme 2; biomarker; diastolic heart failure; hypertension; renin–angiotensin–aldosterone system; systolic heart failure.
© The Author(s) 2016.
Conflict of interest statement
Declaration of conflicting interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Figure 1.
Flow diagram of subject selection. Beside a control group of healthy individuals without any cardiovascular pathology (_n_=45) hypertensive patients (_n_=239), patients with heart failure with reduced ejection fraction (HFrEF, _n_=141) and patients with heart failure with preserved ejection fraction (HFpEF, _n_=47) were enrolled for biochemical and echocardiographic analyses.
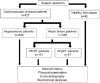
Figure 2.
Serum ACE2 activity parallels cardiovascular disease development. ACE2 activity was measured in the sera of hypertensive patients with heart failure with reduced ejection fraction (HFrEF, _n_=102), hypertensive patients with heart failure with preserved ejection fraction (HFpEF, _n_=47) and patients with HFrEF without hypertension (_n_=39). ACE2 activity is expressed as U/ml of serum, where 1 unit (U) corresponds to 0.1 nmol Mca-APK(Dnp) cleavage in 1 hour at 37°C. Bars represent the mean and SEM. Statistical significance was tested by the nonparametric Kruskal–Wallis test among the groups. Correlation was considered to be significant when p<0.05. There is no statistical difference between values for HFrEF patients with or without hypertension, which is shown by NS.
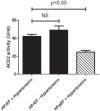
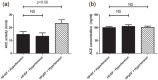
Figure 3.
Comparisons of serum ACE activities and serum ACE concentrations between heart failure cohorts. Serum ACE activity (panel (a)) and ACE concentration (panel (b)) were measured in hypertensive patients with heart failure with reduced ejection fraction (HFrEF, _n_=102), hypertensive patients with heart failure with preserved ejection fraction (HFpEF, _n_=47) and patients with HFrEF without hypertension (_n_=39). Statistical analyses of biochemical measurements were performed by one-way analysis of variance (ANOVA) followed by nonparametric Kruskal–Wallis test among groups. Bars represent mean ± SEM. Correlation was considered to be significant when p<0.05. Lack of statistical difference is labelled by NS.
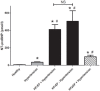
Figure 4.
Serum amino-terminal pro-B-type natriuretic peptide (NT-proBNP) concentration parallels cardiovascular disease development. NT-proBNP concentration was measured in the sera of healthy (_n_=45) individuals and hypertensive patients without signs of heart failure (_n_=239), hypertensive patients with heart failure with reduced ejection fraction (HFrEF, _n_=102), hypertensive patients with heart failure with preserved ejection fraction (HFpEF, _n_=47) and patients with HFrEF without hypertension (_n_=39). NT-proBNP concentration is expressed as pmol/l. Bars represent the mean and SEM. Statistical significance was tested by the nonparametric Kruskal–Wallis test among the groups. Asterisks show statistical difference from the healthy and hash tags show statistical difference from the hypertensive group. There is no statistical difference between values for HFrEF patients with or without hypertension, which is shown by NS.
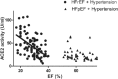
Figure 5.
Serum ACE2 activity correlates with the severity of systolic dysfunction. Serum ACE2 activity (expressed as U/ml of serum) of hypertensive patients with heart failure with reduced EF (HFrEF, _n_=141) and of hypertensive patients with heart failure with preserved EF (HFpEF, _n_=47) is shown as a function of the ejection fraction (EF). Serum ACE2 activity and EF negatively correlated (_r_2=0.26 and p<0.001, slope −2.1±0.36) in the HFrEF group. There was no correlation (defined as _r_2>0.1 and p<0.05) among these parameters in the healthy, hypertensive and HFpEF groups. Data of the healthy and hypertensive groups have been published earlier.
Figure 6.
There is no correlation between serum ACE2 activity and the severity of diastolic dysfunction. There was no correlation between serum ACE2 activity (expressed as U/ml serum) and left ventricular diastolic parameters (E/A, _n_=30, panel (a) and E/e’, _n_=43, panel (b)) of patients with heart failure with preserved ejection fraction (HFpEF). E/A values were determined in HFpEF patients without atrial fibrillation. E/e’ values were determined in patients with sufficient acoustic window for accurate echocardiographic measurement. The threshold for correlation was defined as _r_2>0.1 and p<0.05.
Figure 7.
Comparison of the prognostic value for serum ACE2 activity and amino-terminal pro-B-type natriuretic peptide (NT-proBNP) concentration to differentiate HFrEF and HFpEF from hypertension. Receiver operating characteristic (ROC) curves were generated to test the diagnostic value of serum ACE2 activity (panels (a) and (b)) or NT-proBNP (panels (c) and (d)) to differentiate between patients with heart failure and hypertension without heart failure. Results for HFrEF vs. hypertensive are shown on panels (a) and (c), results for HFpEF vs. hypertensive are shown on panels (b) and (d). Parameters of the ROC analysis are shown as inserts in the plots.
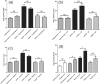
Figure 8.
Effects of comorbidities on serum ACE2 activity in cardiovascular patients. The effects of comorbidities such as dyslipidemia (panel (a)), atrial fibrillation (AF, panel (b)), diabetes (panel (c)) and gender (panel (d)) are shown in cardiovascular patients (healthy, hypertensive without heart failure, heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF)). Statistical differences between patient groups with and without of the defined comorbidity are indicated: asterisks represent statistically significant differences, NS represents no significant statistical difference. Statistical analysis was made by a nonparametric test (Kruskal–Wallis).
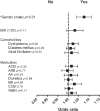
Figure 9.
Logistic regression analyses for confounding variables such as gender, elevated BMI values, cardiovascular comorbidities and cardiovascular drug therapy in hypertensive patients. Male gender has a positive predictive value for elevation of ACE2 activities (p<0.01), no other clinical parameter (BMI>25, presence of diabetes mellitus, dyslipidemia or atrial fibrillation) and no type of the investigated cardiovascular medication (angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), beta blockers (BB), aldosterone antagonists (AA), diuretics, calcium-channel blockers (CCB), statins) have a predictive value for changes of ACE2 activities in hypertensive patients.
Similar articles
- New perspectives in the renin-angiotensin-aldosterone system (RAAS) IV: circulating ACE2 as a biomarker of systolic dysfunction in human hypertension and heart failure.
Úri K, Fagyas M, Mányiné Siket I, Kertész A, Csanádi Z, Sándorfi G, Clemens M, Fedor R, Papp Z, Édes I, Tóth A, Lizanecz E. Úri K, et al. PLoS One. 2014 Apr 1;9(4):e87845. doi: 10.1371/journal.pone.0087845. eCollection 2014. PLoS One. 2014. PMID: 24691269 Free PMC article. - [Role of ACE2-Ang (1-7)-Mas receptor axis in heart failure with preserved ejection fraction with hypertension].
Yu J, Wu Y, Zhang Y, Zhang L, Ma Q, Luo X. Yu J, et al. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018 Jul 28;43(7):738-746. doi: 10.11817/j.issn.1672-7347.2018.07.007. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018. PMID: 30124209 Chinese. - Biomarker Profiles in Heart Failure Patients With Preserved and Reduced Ejection Fraction.
Tromp J, Khan MA, Klip IT, Meyer S, de Boer RA, Jaarsma T, Hillege H, van Veldhuisen DJ, van der Meer P, Voors AA. Tromp J, et al. J Am Heart Assoc. 2017 Mar 30;6(4):e003989. doi: 10.1161/JAHA.116.003989. J Am Heart Assoc. 2017. PMID: 28360225 Free PMC article. - Role of the ACE2/Angiotensin 1-7 Axis of the Renin-Angiotensin System in Heart Failure.
Patel VB, Zhong JC, Grant MB, Oudit GY. Patel VB, et al. Circ Res. 2016 Apr 15;118(8):1313-26. doi: 10.1161/CIRCRESAHA.116.307708. Circ Res. 2016. PMID: 27081112 Free PMC article. Review. - A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation.
Paulus WJ, Tschöpe C. Paulus WJ, et al. J Am Coll Cardiol. 2013 Jul 23;62(4):263-71. doi: 10.1016/j.jacc.2013.02.092. Epub 2013 May 15. J Am Coll Cardiol. 2013. PMID: 23684677 Review.
Cited by
- Persistently Increased Systemic ACE2 Activity Is Associated With an Increased Inflammatory Response in Smokers With COVID-19.
Kaur G, Yogeswaran S, Muthumalage T, Rahman I. Kaur G, et al. Front Physiol. 2021 May 28;12:653045. doi: 10.3389/fphys.2021.653045. eCollection 2021. Front Physiol. 2021. PMID: 34122129 Free PMC article. - Insights into Cardiovascular Defects and Cardiac Epigenome in the Context of COVID-19.
Sarkar S, Sen R. Sarkar S, et al. Epigenomes. 2022 Apr 21;6(2):13. doi: 10.3390/epigenomes6020013. Epigenomes. 2022. PMID: 35645252 Free PMC article. Review. - Quantifying Renin-Angiotensin-System Alterations in COVID-19.
Pucci F, Annoni F, Dos Santos RAS, Taccone FS, Rooman M. Pucci F, et al. Cells. 2021 Oct 14;10(10):2755. doi: 10.3390/cells10102755. Cells. 2021. PMID: 34685735 Free PMC article. Review. - Coronavirus Disease 2019 (COVID-19): Do Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers Have a Biphasic Effect?
Sommerstein R, Kochen MM, Messerli FH, Gräni C. Sommerstein R, et al. J Am Heart Assoc. 2020 Apr 7;9(7):e016509. doi: 10.1161/JAHA.120.016509. Epub 2020 Apr 1. J Am Heart Assoc. 2020. PMID: 32233753 Free PMC article. No abstract available. - Risks of ACE Inhibitor and ARB Usage in COVID-19: Evaluating the Evidence.
Sriram K, Insel PA. Sriram K, et al. Clin Pharmacol Ther. 2020 Aug;108(2):236-241. doi: 10.1002/cpt.1863. Epub 2020 May 10. Clin Pharmacol Ther. 2020. PMID: 32320478 Free PMC article. Review.
References
- Dzau V, Braunwald E. Resolved and unresolved issues in the prevention and treatment of coronary artery disease: A workshop consensus statement. Am Heart J 1991; 121: 1244–1263. - PubMed
- Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet 2007; 370: 591–603. - PubMed
- McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012; 14: 803–869. - PubMed
- Ho KK, Pinsky JL, Kannel WB, et al. The epidemiology of heart failure: The Framingham Study. J Am Coll Cardiol 1993; 22: 6A–13A. - PubMed
- Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008; 359: 2456–2467. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous