Toxic PRn poly-dipeptides encoded by the C9orf72 repeat expansion block nuclear import and export - PubMed (original) (raw)
Toxic PRn poly-dipeptides encoded by the C9orf72 repeat expansion block nuclear import and export
Kevin Y Shi et al. Proc Natl Acad Sci U S A. 2017.
Abstract
The toxic proline:arginine (PRn) poly-dipeptide encoded by the (GGGGCC)n repeat expansion in the C9orf72 form of heritable amyotrophic lateral sclerosis (ALS) binds to the central channel of the nuclear pore and inhibits the movement of macromolecules into and out of the nucleus. The PRn poly-dipeptide binds to polymeric forms of the phenylalanine:glycine (FG) repeat domain, which is shared by several proteins of the nuclear pore complex, including those in the central channel. A method of chemical footprinting was used to characterize labile, cross-β polymers formed from the FG domain of the Nup54 protein. Mutations within the footprinted region of Nup54 polymers blocked both polymerization and binding by the PRn poly-dipeptide. The aliphatic alcohol 1,6-hexanediol melted FG domain polymers in vitro and reversed PRn-mediated enhancement of the nuclear pore permeability barrier. These data suggest that toxicity of the PRn poly-dipeptide results in part from its ability to lock the FG repeats of nuclear pore proteins in the polymerized state. Our study offers a mechanistic interpretation of PRn poly-dipeptide toxicity in the context of a prominent form of ALS.
Keywords: C9orf72 repeat expansion; FG domain; PRn poly-dipeptide; labile cross-β polymers; nuclear pore.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
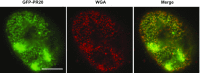
Fig. S1.
Confocal microscopy image of GFP-PR20 and WGA binding to nuclear pores of U2OS cells. U2OS cells grown in a glass-bottom dish were permeabilized in CSK buffer (10 mM Pipes pH 7.0, 100 mM NaCl, 300 mM sucrose, and 3 mM MgCl2) supplemented with Triton X-100 (0.7%). Permeabilized cells were incubated with GFP-PR20 and Alexa Fluor 555-WGA in PBS. Images were obtained with a Leica confocal microscope. GFP-PR20 colocalized with nuclear pores marked by WGA. (Scale bar: 5 μm.)
Fig. 1.
STED images of PR20 binding to nuclear pores of X. laevis oocyte germinal vessicles. (A) FITC-labeled PR20 peptide (green) bound to the nuclear envelope in a punctate pattern, but individual pores were not resolvable by confocal microscopic imaging. (B) The same field as in A viewed by STED microscopy. Individual nuclear pores were thus resolved. In some pores, PR20 peptide binding appeared annular (arrows). (C) Graph comparing sizes of 250 annuli labeled with an antibody against the nucleoporin gp210 or with the PR20 peptide, with dimensions similar to those described by Löschberger et al. (15) and Göttfert et al. (40). (D–F) WGA-Alexa Fluor 555 (pseudocolored green) labeled the central channel of nuclear pores, whereas antibody against nucleoporin gp210 (red) labeled the pore periphery in an annular pattern. (G–I) Atto 647-PR20 (pseudocolored green) labeled the central channel, whereas an antibody against nucleoporin gp210 (red) labeled the periphery of nuclear pores.
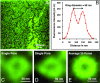
Fig. 2.
Ring diameter of PR20 poly-dipetide localization is consistent with the diameter of the central channel of the nuclear pore complex. (A) A portion of unfixed X. laevis oocyte nuclear envelope stained with Atto647N-PR20 peptide and imaged with the STED 775-nm laser. Only single stained envelopes were used for quantification, because these offered the highest resolution. (B) An average line profile plot of the image in E was generated in FIJI (ImageJ) and exported to MS Excel. The line was placed diagonally across the region of interest and rotated eight times using a custom macro script. (C and D) Magnification of two example single pores from the 25 pores selected for analysis shown by the white circles in A. (E) The 25 single pores selected in A were aligned into a stack and then averaged in FIJI using a custom macro script. Ten such “average” pores from 10 different images were used to determine the average ring diameter shown in Fig. 1_C_. Pores were hand-selected to ensure that the analysis was conducted only on pores with a visible ring structure.
Fig. 3.
PR20 poly-dipeptides impede the nuclear export and import of macromolecules. (A) U2OS cells were exposed to 2.5, 5, or 10 μM concentrations of a synthetic peptide containing 20 repeats of the PR poly-dipeptide (PR20). Then, 8 h later, the cells were fixed and probed for polyA+ RNA by in situ hybridization. Cells exposed to PR20 retained polyA+ RNA in the nucleus in a speckled pattern. Quantitation of >50 cells per dose (Right) shows the relative intensity of the in situ hybridization signal in the cytoplasmic and nuclear compartments as a function of PR20 dose. (B) Diagram of a U2OS cell stably expressing GFP fused to both nuclear import and export signals. (Left) Under control conditions, most of the GFP accumulates in the cytoplasm. (Right) Leptomycin B (LMB) inhibits nuclear export, causing GFP to accumulate in the nucleus. The concentration of GFP in the nucleus, measured by fluorescence intensity, rose linearly as a function of time after the addition of LMB (control). Preincubating cells with PR20 poly-dipeptide inhibited the accumulation of GFP in the nucleus in a dose-dependent manner (2.5–10 μM). (C) HeLa cells were permeabilized with digitonin and exposed to concentrated HeLa cytoplasm along with fluorescein-labeled BSA coupled to a nuclear localization signal. Such cells accumulated BSA in the nucleus (control). Addition of the toxic PR20 poly-dipeptide prevented the nuclear import of BSA, which accumulated in the cytoplasm in a dose-dependent manner (0.5–5 μM). (D) Graph showing the concentration of labeled BSA in the nucleus (y axis) as a function of PR20 concentration in the medium (x axis).
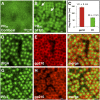
Fig. S2.
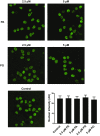
PA20 and PG20 poly-dipeptides do not impede the import of labeled BSA into HeLa cell nuclei. HeLa cells grown on glass coverslips were permeabilized with digitonin, then exposed to concentrated HeLa cytosol supplemented with fluorescent-tagged BSA conjugated to a nuclear localization peptide. The labeled BSA was observed to partition into nuclei (Bottom Left) in a manner not impeded by 2.5 or 5 μM concentrations of either PA20 (Top) or PG20 (Middle). (Bottom Right) The intensity of nuclear fluorescence was quantitated in >50 cells for each condition.
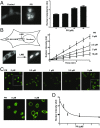
Fig. 4.
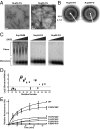
Characterization of FG repeat domain polymers formed by Nup54 and Nup98. (A) Transmission electron micrographs of polymeric fibers of the FG domains of Nup54 and Nup98 negatively stained with uranyl acetate. (Scale bar: 200 nm.) (B) X-ray diffraction patterns of lyophilized Nup54 and Nup98 FG domain polymers. (C) SDD-AGE of amyloid polymers formed by yeast Sup35NM, Nup54 FG domain, or Nup98 FG domain. Incubation with increasing amounts of SDS did not substantially affect ySup35NM aggregates. Both Nup54 and Nup98 fibers were fully depolymerized under similar conditions. (D) Chemical footprinting of Nup54 fibers using the acetylation reagent NAI, as analyzed by SILAC mass spectrometry. The reactivity of specific residues in Nup54 to NAI was compared in the fully polymerized state and the denatured state. The degree of protection from acetylation on polymerization is reflected by the ratio of acetylation in denatured/native conditions (y axis). (E) WT or phenylalanine-to-proline double-mutant Nup54 monomers were incubated with WT Nup54 polymer seeds. An increase in the fluorescence of thioflavin-T (y axis) as a function of time (x axis) reflects seeded polymerization of Nup54 monomers.
Fig. 5.
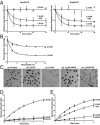
PR20 poly-dipeptide binds to the FG repeat domain of Nup54 and Nup98. (A) A region encoding the FG repeats of Nup54 was expressed in bacterial cells, purified, and incubated under conditions leading to its polymerization. Monomeric and polymeric forms of the FG domain were exposed to dye-labeled PR20 poly-dipeptide and monitored by fluorescence polarization. Fluorescence anisotropy (y axis) was measured as a function of the concentration of Nup54 FG domain (x axis). Only the polymeric sample led to an increase in fluorescence anisotropy. (B) Addition of unlabeled PR20 peptide, but not PA20 or PG20 poly-dipeptides, displaced binding of dye-labeled PR20 peptide to the polymeric form of the FG domain of Nup54. (C) Monomeric and polymeric forms of the Nup98 FG domain were exposed to dye-labeled PR20 poly-dipeptide and monitored by fluorescence polarization. Fluorescence anisotropy (y axis) was measured as a function of the concentration of Nup98 FG domain (x axis). Only the polymeric sample led to an increase in fluorescence anisotropy. (D) The addition of unlabeled PR20 peptide, but not of PA20 or PG20 poly-dipeptides, displaced binding of the dye-labeled PR20 poly-dipeptide to Nup98 FG domain polymers. (E) WT or F39P/F41P mutant monomers of the Nup54 FG domain were incubated with WT polymeric seeds along with dye-labeled PR20. Fluorescence anisotropy (y axis) was measured as a function of the time (x axis). Only the seeded reaction supplemented with WT monomers led to an increase in fluorescence anisotropy. (F) Fluorescence micrographs of dye-labeled PR20 poly-dipeptide bound to polymeric fibers of the FG domains of Nup54 and Nup98. (Scale bar: 20 µm.)
Fig. 6.
Effect of aliphatic alcohols on FG domain polymers, PR20 binding to FG domain polymers, and nuclear partitioning of 70-kDa fluorescent dextran. (A) Polymeric fibers of the FG domains of Nup54 and Nup98 were exposed to 1,6-HD or 2,5-HD (15%), and polymer melting was assayed by a decrease in light scattering at 395 nm. Polymers were unaffected by 2,5-HD, but melted over a period of 30 min by 1,6-HD. Polymer melting by 1,6-HD was partially inhibited in the presence of the PR20 poly-dipeptide (20 µM). (B) Binding of dye-labeled PR20 poly-dipeptide to FG domain polymers was measured by fluorescence anisotropy as a function of alcohol concentration. 1,6-HD reduced PR20 binding more effectively than 2,5-HD. (C) Digitonin-permeabilized HeLa cells were exposed to 70-kDa dextran coupled to a fluorescent dye (Texas Red). (a) The fluorescent dextran was excluded from the nuclei of control cells (dark areas). (b) 2,5-HD did not affect movement of the fluorescent dextran into nuclei. (c) 1,6-HD facilitated entry of the fluorescent dextran into nuclei. (d) WGA (0.8 mg/mL) impeded 1,6-HD–mediated entry of the fluorescent dextran into nuclei. (e) PR20 peptide (20 µM) impeded 1,6-HD–mediated entry of the fluorescent dextran into nuclei. All images correspond to maximal uptake in 4 min. (D) Quantitation of the entry of fluorescent dextran into nuclei (y axis) as a function of time (x axis) after the addition of either 1,6-HD or 2,5-HD. (E) Quantitation of fluorescent dextran entry into nuclei (y axis) as a function of time (x axis) after the addition of 1,6-HD in the presence of the PR20 peptide or WGA. The curves for the effect of 1,6-HD on dextran uptake in D and E are the same data plotted in two graphs.
Comment in
- A PR plug for the nuclear pore in amyotrophic lateral sclerosis.
Taylor JP. Taylor JP. Proc Natl Acad Sci U S A. 2017 Feb 14;114(7):1445-1447. doi: 10.1073/pnas.1621085114. Epub 2017 Feb 3. Proc Natl Acad Sci U S A. 2017. PMID: 28159889 Free PMC article. No abstract available.
Similar articles
- Toxic PR Poly-Dipeptides Encoded by the C9orf72 Repeat Expansion Target LC Domain Polymers.
Lin Y, Mori E, Kato M, Xiang S, Wu L, Kwon I, McKnight SL. Lin Y, et al. Cell. 2016 Oct 20;167(3):789-802.e12. doi: 10.1016/j.cell.2016.10.003. Cell. 2016. PMID: 27768897 Free PMC article. - Synthetic hydrogel mimics of the nuclear pore complex for the study of nucleocytoplasmic transport defects in C9orf72 ALS/FTD.
Friedman AK, Boeynaems S, Baker LA. Friedman AK, et al. Anal Bioanal Chem. 2022 Jan;414(1):525-532. doi: 10.1007/s00216-021-03478-2. Epub 2021 Jun 25. Anal Bioanal Chem. 2022. PMID: 34170347 - The DNA damage response (DDR) is induced by the C9orf72 repeat expansion in amyotrophic lateral sclerosis.
Farg MA, Konopka A, Soo KY, Ito D, Atkin JD. Farg MA, et al. Hum Mol Genet. 2017 Aug 1;26(15):2882-2896. doi: 10.1093/hmg/ddx170. Hum Mol Genet. 2017. PMID: 28481984 - Pathogenesis underlying hexanucleotide repeat expansions in C9orf72 gene in amyotrophic lateral sclerosis.
Chong ZZ, Menkes DL, Souayah N. Chong ZZ, et al. Rev Neurosci. 2023 Aug 2;35(1):85-97. doi: 10.1515/revneuro-2023-0060. Print 2024 Jan 29. Rev Neurosci. 2023. PMID: 37525497 Review. - There has been an awakening: Emerging mechanisms of C9orf72 mutations in FTD/ALS.
Gitler AD, Tsuiji H. Gitler AD, et al. Brain Res. 2016 Sep 15;1647:19-29. doi: 10.1016/j.brainres.2016.04.004. Epub 2016 Apr 6. Brain Res. 2016. PMID: 27059391 Free PMC article. Review.
Cited by
- Phase separation of low-complexity domains in cellular function and disease.
Lee J, Cho H, Kwon I. Lee J, et al. Exp Mol Med. 2022 Sep;54(9):1412-1422. doi: 10.1038/s12276-022-00857-2. Epub 2022 Sep 29. Exp Mol Med. 2022. PMID: 36175485 Free PMC article. Review. - Multifaceted Genes in Amyotrophic Lateral Sclerosis-Frontotemporal Dementia.
Ranganathan R, Haque S, Coley K, Shepheard S, Cooper-Knock J, Kirby J. Ranganathan R, et al. Front Neurosci. 2020 Jul 7;14:684. doi: 10.3389/fnins.2020.00684. eCollection 2020. Front Neurosci. 2020. PMID: 32733193 Free PMC article. Review. - Molecular Tweezers: Supramolecular Hosts with Broad-Spectrum Biological Applications.
Shahpasand-Kroner H, Siddique I, Malik R, Linares GR, Ivanova MI, Ichida J, Weil T, Münch J, Sanchez-Garcia E, Klärner FG, Schrader T, Bitan G. Shahpasand-Kroner H, et al. Pharmacol Rev. 2023 Mar;75(2):263-308. doi: 10.1124/pharmrev.122.000654. Epub 2022 Dec 22. Pharmacol Rev. 2023. PMID: 36549866 Free PMC article. Review. - Single-molecule imaging reveals distinct elongation and frameshifting dynamics between frames of expanded RNA repeats in C9ORF72-ALS/FTD.
Latallo MJ, Wang S, Dong D, Nelson B, Livingston NM, Wu R, Zhao N, Stasevich TJ, Bassik MC, Sun S, Wu B. Latallo MJ, et al. Nat Commun. 2023 Sep 11;14(1):5581. doi: 10.1038/s41467-023-41339-x. Nat Commun. 2023. PMID: 37696852 Free PMC article. - p53 Transactivation Domain Mediates Binding and Phase Separation with Poly-PR/GR.
Usluer S, Spreitzer E, Bourgeois B, Madl T. Usluer S, et al. Int J Mol Sci. 2021 Oct 22;22(21):11431. doi: 10.3390/ijms222111431. Int J Mol Sci. 2021. PMID: 34768862 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous