Dendritic cell MST1 inhibits Th17 differentiation - PubMed (original) (raw)
doi: 10.1038/ncomms14275.
Yujing Bi 3, Yan Li 1 2, Hui Yang 2, Qing Yu 1, Jian Wang 1 2, Yu Wang 1 2, Huilin Su 1 2, Anna Jia 1, Ying Hu 1, Linian Han 1, Jiangyuan Zhang 1, Simin Li 1, Wufan Tao 4, Guangwei Liu 1 2
Affiliations
- PMID: 28145433
- PMCID: PMC5296641
- DOI: 10.1038/ncomms14275
Dendritic cell MST1 inhibits Th17 differentiation
Chunxiao Li et al. Nat Commun. 2017.
Abstract
Although the differentiation of CD4+T cells is widely studied, the mechanisms of antigen-presenting cell-dependent T-cell modulation are unclear. Here, we investigate the role of dendritic cell (DC)-dependent T-cell differentiation in autoimmune and antifungal inflammation and find that mammalian sterile 20-like kinase 1 (MST1) signalling from DCs negatively regulates IL-17 producing-CD4+T helper cell (Th17) differentiation. MST1 deficiency in DCs increases IL-17 production by CD4+T cells, whereas ectopic MST1 expression in DCs inhibits it. Notably, MST1-mediated DC-dependent Th17 differentiation regulates experimental autoimmune encephalomyelitis and antifungal immunity. Mechanistically, MST1-deficient DCs promote IL-6 secretion and regulate the activation of IL-6 receptor α/β and STAT3 in CD4+T cells in the course of inducing Th17 differentiation. Activation of the p38 MAPK signal is responsible for IL-6 production in MST1-deficient DCs. Thus, our results define the DC MST1-p38MAPK signalling pathway in directing Th17 differentiation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
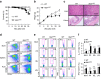
Figure 1. DC MST1 deficiency leads to spontaneous diseases.
(a) Kaplan–Meier plots of WT and _Mst1_ΔDC mouse survival after birth are shown (_n_=20). (b) Body weights of the WT and _Mst1_ΔDC mice after birth are summarized (_n_=20). (c) H&E staining of hepatic and intestinal tissues from sex- and age-matched WT and _Mst1_ΔDC mice at 20 weeks after birth is presented. Scale bars, 100 μm. (d) DC MST1 deficiency leads to more CD44highCD62Llow cells among CD4+T cells than WT control mice at 20 weeks after birth. Gating strategies for determining the percentage of CD4+TCR+CD44highCD62Llow cells are showed in Supplementary Fig. 4C. Intracellular IL-17A and IFN-γ expression of CD4+T cells isolated from MLN, PP, IEL and LPL in WT and _Mst1_ΔDC mice with a typical figure displayed (e) and the frequencies of positive cells summarized (f). Data are representative of three to four independent experiments (mean±s.d.; _n_=4). ***P<0.001, compared with the indicated groups. n.s., not significant. _P_-values were determined using Student's _t_-tests.
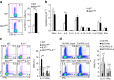
Figure 2. DC MST1 inhibits Th17 differentiation in EAE.
(a) EAE disease course in WT and _Mst1_ΔDC mice. Intercellular staining of IL-17+ cells, IFNγ+ cells, IL-4+ cells (after stimulation with PMA and ionomycin) and Foxp3+ cells among CD4+T cells from the spinal cord of WT or _Mst1_ΔDC mice were determined with flow cytometry method (FCM) on day 19 after MOG immunization. A typical figure is shown (b) and the data summarized (c). (d) CD4+T cells isolated from spinal cord of _Mst1_ΔDC mice on the indicated day after MOG immunization along with mRNA expression of the indicated gene (levels in WT groups were set to 1). (e,f) CD4+T cells isolated from dLN of MOG-immunized WT or _Mst1_ΔDC mice were labelled with CFSE and stimulated with MOG for 5 days. Intercellular staining of IL-17 and IFNγ in CFSElow cells was determined with FCM. The typical figure is shown in e and data summarized (f). (g,h) CD4+T cells isolated from the dLN of MOG-immunized WT or _Mst1_ΔDC mice, and ex vivo stimulation with anti-CD3 (1 μg ml−1) for 24 h and mRNA expression (g) or cytokine secretion (h) of the indicated gene were analysed using qPCR (levels in the WT groups were set to 1) or ELISA. Data are representative of three to four independent experiments (mean±s.d.; _n_=4–6). *P<0.05 and ***P<0.001, compared with the indicated groups. _P_-values were determined using Student's _t_-tests.
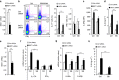
Figure 3. DC MST1 inhibits Th17 differentiation in anti-fungi infection.
Mice were infected with 105 C. albicans SC5314 by i.v. injection. After 9 days, kidneys were collected and a photo of the H&E staining of pathological kidney injuries is shown (Scale bars, 100 μm) (a) and the fungal burden in the kidneys expressed as CFU per g (b). (c) Serum cytokine level of IL-17 and IFNγ in infected mice. (d,e) CD4+T cells isolated from the spleen of the WT or _Mst1_ΔDC mice were labelled with CFSE and stimulated with heat-killed C. albicans for 3 days. Intracellular staining of IL-17 and IFNγ in CFSElow cells was determined with FCM. A typical figure is shown (d) and the data summarized (e). (f) CD4+T cells isolated from the spleen of infected WT or _Mst1_ΔDC mice, and ex vivo stimulation was performed with anti-CD3 (1 μg ml−1) for 24 h, then mRNA expression of the indicated gene was analysed using qPCR (Levels in the WT groups were set to 1). Data are representative of three to four independent experiments (mean±s.d.; _n_=4–8). *P<0.05 and ***P<0.001, compared with the indicated groups. _P_-values were determined using Student's _t_-tests.
Figure 4. DC MST1 signalling directs Th17 differentiation in vivo.
(a) WT or _Mst1_ΔDC mice were immunized with OVA+CFA for 7–8 days. The T cells were isolated from the dLN and restimulated ex vivo for 3 days with OVA (5 μg ml−1) in the presence of irradiated splenocytes, and the secretion of indicated cytokines were analysed by ELISA. (b–d) Naive MOG-transgenic 2D2 T cells (Thy1.1+) were transferred into WT or _Mst1_ΔDC mice and immunized with MOG+CFA for 7–8 days. (b) Proliferation of dLN cells was determined with 3H-TdR incorporation. (c) Expression levels of IL-17A and IFNγ in donor cells in dLN. Right, proportion of IL-17A and IFNγ in donor cells. (d) Secretion of IL-17 and IFNγ in donor cells stimulated with MOG for 72 h. (e,f) Naive OT-II T cells (Thy1.1+) were transferred into WT or _Mst1_ΔDC mice and immunized with OVA+CFA for 7–8 days. (e) Proliferation of dLN cells was determined with 3H-TdR incorporation. (f) Expressions of IL-17A and IFNγ in donor cells in the dLN. Right, proportion of IL-17A and IFNγ in donor cells. (g) mRNA expression of IL-17 and IFNγ in donor cells stimulated with OVA for 72 h. Levels in WT control groups were set to 1. Data are representative of three to four independent experiments (mean±s.d.; _n_=3–6). ***P<0.001, compared with the indicated groups. _P_-values were determined using Student's _t_-tests.
Figure 5. DC MST1 signalling instructs Th17 differentiation in vitro.
(a) Naive CD4+T cells sorted from OT-II mice stimulated with antigen and LPS-pulsed CD8+DCs or CD11b+DCs from WT or _Mst1_ΔDC mice for 5 days. Intercellular staining of IL-17 and IFNγ in T cells. Right, proportion of IL-17 and IFNγ in T cells. (b) Naive CD4+T cells sorted from C57BL/6 mice stimulated with anti-CD3 (2 μg ml−1) and LPS-pulsed CD8+DCs or CD11b+DCs from WT or _Mst1_ΔDC mice for 5 days. Intercellular staining of IL-17 and IFNγ in T cells was determined with FCM and a typical figure is shown. Right, proportion of IL-17 and IFNγ in T cells. (c) Naive T cells from C57BL/6 mice were co-cultured with WT or _Mst1_ΔDC splenic DCs for 5 days in the presence of anti-CD3 and LPS. mRNA expression of the indicated genes in T cells were determined with qPCR. Levels in the WT control groups were set to 1. Data are representative of three to four independent experiments (mean±s.d.; _n_=4–6). **P<0.01 and ***P<0.001, compared with the indicated groups. _P_-values were determined using Student's _t_-tests.
Figure 6. MST1 controls DC IL-6 production during Th17 differentiation.
(a) Intracellular staining of IL-6 in WT or _Mst1_ΔDC splenic DCs 5 h after LPS stimulation in vitro. (Right) Proportion of IL-6 among the CD11c+ cells. The supernatant was collected and cytokine secretions determined with ELISA (b). (c,d) CD4+T cells from the indicated mice stimulated with WT or _Mst1_ΔDC splenic DCs for 5 days. Intercellular staining of IL-17 and IFNγ in T cells was determined with FCM and a typical figure is shown. Right, proportion of IL-17 and IFNγ in T cells. Data are representative of three to four independent experiments (mean±s.d.; _n_=4–5). ***P<0.001, compared with the indicated groups. n.s., not significant. _P_-values were determined using Student's _t_-tests.
Figure 7. T-cell IL-6R-p-STAT3 is responsible for DC MST1 signalling.
(a) Expression of IL-6Rα and IL-6Rβ (gp130) in T cells co-cultured with WT or _Mst1_ΔDC splenic DCs for 3 days. Right, the mean fluorescent intensity (MFI) is summarized. (b) Intercellular staining of p-STAT3 and p-STAT4 in T cells co-cultured with WT or _Mst1_ΔDC splenic DCs for 3 days. Right, MFI of the indicated protein is summarized. (c,d) Sorted CD4+ T cells were transfected with control or IL-6Rα (c) and IL-6Rβ (d) siRNA vector and stimulated with WT or _Mst1_ΔDC splenic DCs for 5 days. The intercellular staining of IL-17 and IFNγ in T cells performed with FCM and a typical figure shown. Right, the proportion of IL-17 and IFNγ in T cells. Data are representative of three to four independent experiments (mean±s.d.; _n_=4). *P<0.05, **P<0.01 and ***P<0.001, compared with the indicated groups. n.s., not significant. _P_-values were determined using Student's _t_-tests.
Figure 8. MST1 regulates IL-6 production through p38MAPK.
(a) Intercellular staining of the indicated proteins in splenic DCs following LPS stimulation in vitro. Expression of the phosphorylation of p38MAPK (MFI) among CD11c+cells is summarized in b. (c,d) Intracellular staining of IL-6 in DC isolated from the indicated mice 4 h after the i.p. injection of 10 mg kg−1 LPS (c). Right, the proportion of IL-6+ cells in DCs is summarized. The serum IL-6 level in the indicated mice (d). (e) Intracellular staining of IL-17 and IFNγ in T cells co-cultured with splenic DCs isolated from indicated mice was determined with FCM. Right, proportion of IL-17 and IFNγ in CD4+T cells is summarized. (f) Expression of the indicated gene in T cells was determined with FCM and the MFI summarized. Data are representative of four independent experiments (mean±s.d.; _n_=3–5). *P<0.05, **P<0.01 and ***P<0.001, compared with the indicated groups. _P_-values were determined using Student's _t_-tests.
Figure 9. Mouse and human DC MST1 modulates T-cell differentiation.
Mouse (a) or human (e) DCs transfected with MST1-specfic or control siRNA and mRNA of indicated gene were determined with qPCR. Levels in the control groups were set to 1. Mouse DCs (b–d) or human DCs (f–h) pulsed with LPS (100 ng ml−1) were co-cultured with mouse or human T cells, respectively. The expression of IL-17 and IFNγ in T cells was determined with FCM (b) or qPCR (f); MFI of the indicated gene in T cells (c) and mRNA of the indicated genes in T cells (g); the levels of IL-6 in the indicated supernatant (d,h) were determined. Data are representative of three independent experiments (mean±s.d.; _n_=4–6). *P<0.05 and ***P<0.001, compared with the indicated groups. _P_-values were determined using Student's _t_-tests.
Similar articles
- The MAPK-Activated Kinase MK2 Attenuates Dendritic Cell-Mediated Th1 Differentiation and Autoimmune Encephalomyelitis.
Soukup K, Halfmann A, Le Bras M, Sahin E, Vittori S, Poyer F, Schuh C, Luger R, Niederreiter B, Haider T, Stoiber D, Blüml S, Schabbauer G, Kotlyarov A, Gaestel M, Felzmann T, Dohnal AM. Soukup K, et al. J Immunol. 2015 Jul 15;195(2):541-52. doi: 10.4049/jimmunol.1401663. Epub 2015 Jun 15. J Immunol. 2015. PMID: 26078274 - Betaine Ameliorates Experimental Autoimmune Encephalomyelitis by Inhibiting Dendritic Cell-Derived IL-6 Production and Th17 Differentiation.
Yang C, Lai W, Zhou J, Zheng X, Cai Y, Yang W, Xie S, Gao Y, Du C. Yang C, et al. J Immunol. 2018 Feb 15;200(4):1316-1324. doi: 10.4049/jimmunol.1700920. Epub 2018 Jan 12. J Immunol. 2018. PMID: 29330324 - Stimulation of dopamine receptor D5 expressed on dendritic cells potentiates Th17-mediated immunity.
Prado C, Contreras F, González H, Díaz P, Elgueta D, Barrientos M, Herrada AA, Lladser Á, Bernales S, Pacheco R. Prado C, et al. J Immunol. 2012 Apr 1;188(7):3062-70. doi: 10.4049/jimmunol.1103096. Epub 2012 Feb 29. J Immunol. 2012. PMID: 22379034 - Regulation of TH17 cell differentiation by innate immune signals.
Huang G, Wang Y, Chi H. Huang G, et al. Cell Mol Immunol. 2012 Jul;9(4):287-95. doi: 10.1038/cmi.2012.10. Epub 2012 Apr 16. Cell Mol Immunol. 2012. PMID: 22504954 Free PMC article. Review. - [Immune Regulation by TNF Receptor-associated Factor 5].
So T. So T. Yakugaku Zasshi. 2024;144(5):489-496. doi: 10.1248/yakushi.23-00154-3. Yakugaku Zasshi. 2024. PMID: 38692922 Review. Japanese.
Cited by
- Mammalian STE20‑like kinase 1 regulates pancreatic cancer cell survival and migration through Mfn2‑mediated mitophagy.
Hu Y, Wang B, Wang L, Wang Z, Jian Z, Deng L. Hu Y, et al. Mol Med Rep. 2020 Jul;22(1):398-404. doi: 10.3892/mmr.2020.11098. Epub 2020 Apr 28. Mol Med Rep. 2020. PMID: 32377725 Free PMC article. Retracted. - Aryl hydrocarbon receptor nuclear translocator limits the recruitment and function of regulatory neutrophils against colorectal cancer by regulating the gut microbiota.
Bi Y, Yang Q, Li Z, Wang Y, Wang Y, Jia A, Pan Z, Yang R, Liu G. Bi Y, et al. J Exp Clin Cancer Res. 2023 Mar 1;42(1):53. doi: 10.1186/s13046-023-02627-y. J Exp Clin Cancer Res. 2023. PMID: 36859266 Free PMC article. - Role of Hippo signaling in regulating immunity.
Hong L, Li X, Zhou D, Geng J, Chen L. Hong L, et al. Cell Mol Immunol. 2018 Dec;15(12):1003-1009. doi: 10.1038/s41423-018-0007-1. Epub 2018 Mar 22. Cell Mol Immunol. 2018. PMID: 29568120 Free PMC article. Review. - Regulation of the Hippo pathway in cancer biology.
Moon S, Yeon Park S, Woo Park H. Moon S, et al. Cell Mol Life Sci. 2018 Jul;75(13):2303-2319. doi: 10.1007/s00018-018-2804-1. Epub 2018 Mar 30. Cell Mol Life Sci. 2018. PMID: 29602952 Free PMC article. Review. - CD11c-Specific Deletion Reveals CREB as a Critical Regulator of DC Function during the Germinal Center Response.
Ohl K, Schippers A, Tenbrock K. Ohl K, et al. J Immunol Res. 2018 May 7;2018:8947230. doi: 10.1155/2018/8947230. eCollection 2018. J Immunol Res. 2018. PMID: 29854847 Free PMC article.
References
- Ansel K. M., Lee D. U. & Rao A. An epigenetic view of helper T cell differentiation. Nat. Immunol. 4, 616–623 (2003). - PubMed
- Zhou L., Chong M. M. & Littman D. R. Plasticity of CD4+ T cell lineage differentiation. Immunity 30, 646–655 (2009). - PubMed
- Murphy K. M. & Reiner S. L. The lineage decisions of helper T cells. Nat. Rev. Immunol. 2, 933–944 (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous