Zika Virus Antagonizes Type I Interferon Responses during Infection of Human Dendritic Cells - PubMed (original) (raw)
Zika Virus Antagonizes Type I Interferon Responses during Infection of Human Dendritic Cells
James R Bowen et al. PLoS Pathog. 2017.
Abstract
Zika virus (ZIKV) is an emerging mosquito-borne flavivirus that is causally linked to severe neonatal birth defects, including microcephaly, and is associated with Guillain-Barre syndrome in adults. Dendritic cells (DCs) are an important cell type during infection by multiple mosquito-borne flaviviruses, including dengue virus, West Nile virus, Japanese encephalitis virus, and yellow fever virus. Despite this, the interplay between ZIKV and DCs remains poorly defined. Here, we found human DCs supported productive infection by a contemporary Puerto Rican isolate with considerable variability in viral replication, but not viral binding, between DCs from different donors. Historic isolates from Africa and Asia also infected DCs with distinct viral replication kinetics between strains. African lineage viruses displayed more rapid replication kinetics and infection magnitude as compared to Asian lineage viruses, and uniquely induced cell death. Infection of DCs with both contemporary and historic ZIKV isolates led to minimal up-regulation of T cell co-stimulatory and MHC molecules, along with limited secretion of inflammatory cytokines. Inhibition of type I interferon (IFN) protein translation was observed during ZIKV infection, despite strong induction at the RNA transcript level and up-regulation of other host antiviral proteins. Treatment of human DCs with RIG-I agonist potently restricted ZIKV replication, while type I IFN had only modest effects. Mechanistically, we found all strains of ZIKV antagonized type I IFN-mediated phosphorylation of STAT1 and STAT2. Combined, our findings show that ZIKV subverts DC immunogenicity during infection, in part through evasion of type I IFN responses, but that the RLR signaling pathway is still capable of inducing an antiviral state, and therefore may serve as an antiviral therapeutic target.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
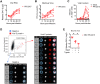
Fig 1. Contemporary Puerto Rican ZIKV isolate productively infects human DCs.
moDCs were infected with ZIKV PR-2015 at MOI of 1 and assessed for viral replication at indicated hours post-infection. (A) Viral RNA was detected in cell lysates by qRT-PCR for ZIKV E protein RNA. Gene expression is shown as relative expression after normalization to GAPDH levels in each respective sample (n = 7 donors). (B) Viral titers in supernatants of ZIKV-infected moDCs as determined by focus forming assay (FFA; n = 8 donors). FFU, focus forming units. (C) Percent infected cells as assessed by ZIKV E protein staining (4G2-APC antibody) and flow cytometry (n = 9 donors). (D) ImageStream analysis of ZIKV-infected moDCs labeled for viral E protein at 48hpi. Images of individual cells highlighted in the flow plot are represented and ordered according to E protein staining intensity. (E) moDCs were infected with ZIKV PR-2015 at MOI of 1 for 1hr on ice, washed extensively, and bound virus was quantitated by qRT-PCR for ZIKV RNA. Gene expression is represented as relative expression after normalization to GAPDH levels in each respective sample and shown as the mean +/- SD from 6–9 donors. See also S1 Fig.
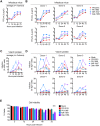
Fig 2. Differential infection of human DCs by evolutionarily distinct ZIKV strains.
moDCs were infected with PR-2015, P6-1966, MR-1947, or Dak-1984 at MOI of 1 and assessed for viral replication at the indicated hours post-infection. (A) Infectious virus release into the supernatant was determined by FFA. Shown as the mean +/- SEM from 6–9 donors. (B) Infectious virus release for 6 of the individual donors summarized in panel A. (C) Percent infected cells assessed by ZIKV E protein staining and flow cytometry. Shown as the mean +/- SEM from 6–9 donors. (D) Percent infected cells in 6 of the individual donors summarized in panel C. (E) Cell viability of infected moDCs assessed by Ghost Red 780 (Tonbo) viability staining and flow cytometry. Shown as the mean +/- SEM from 6–9 donors. Statistical significance (p< 0.05) was determined using a two-way ANOVA with comparisons made to mock-infected cells. See also S1 Table.
Fig 3. ZIKV infection minimally activates human DCs.
(A) moDCs were left uninfected (“Mock”) or infected with PR-2015, P6-1966, MR-1947, or Dak-1984 at MOI of 1 (n = 6–8 donors). Cells were collected at 48hpi and labeled for ZIKV E protein and indicated DC activation markers. Cells were categorized as being viral E protein- or viral E protein+ and activation marker surface expression quantitated by flow cytometry. Values are represented as median fluorescence intensity (MFI) for each individual donor with uninfected and ZIKV infected samples from the same donor connected with a line. Statistical significance (p< 0.05) was determined using a Friedman test with comparisons made to donor-paired, uninfected cells. (B) moDCs infected with PR-2015 at MOI of 1 were stratified into “low” (n = 3 donors) and “high” (n = 5 donors) infection on the basis of viral E protein staining. MFIs are shown as the mean +/- SD. See also S3 Fig.
Fig 4. ZIKV infection induces minimal pro-inflammatory cytokine production by DCs.
(A) moDCs were left untreated (“Mock”), transfected with RIG-I agonist (10ng/1e5 cells), or infected with PR-2015, P6-1966, MR-1947, or Dak-1984 at MOI of 1 (n = 7 donors). Supernatants were collected at 48hpi. (B, C) Monocytes (Mo) and myeloid DCs (mDCs) were left untreated (“Mock”), treated with LPS (100 ng/ml), or infected with PR-2015 at MOI of 1 (n = 5 donors). Supernatants were collected at 24hpi. (D) Plasmacytoid DCs (pDCs) were left untreated (“Mock”), treated with R848 (1 μg/ml), or infected with PR-2015 at MOI of 1 (n = 5 donors). Supernatants were collected at 24hpi. Cytokine production was assessed using multiplex bead array. Values for each individual donor are shown with the mean +/- SD. Statistical significance (p< 0.05) was determined using a Kruskal-Wallis test with comparisons made to untreated (“Mock”) cells. See also S2, S3, S4, and S5 Tables.
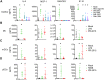
Fig 5. ZIKV infection induces type I IFN transcription but inhibits translation.
moDCs were left untreated (“Mock”), treated with RIG-I agonist (10ng/1e5 cells), or infected with PR-2015, P6-1966, MR-1947, or Dak-1984 at MOI of 1. Supernatants were collected 24hrs (RIG-I agonist treatment) or 48hrs (ZIKV infection) later and IFNβ and IFNα (A) or IFNλ1 (B) production was assessed via multiplex bead array. Values for each individual donor are shown with the mean +/- SD (n = 7 donors). Statistical significance (p< 0.05) was determined using a Friedman test with comparisons made to donor-paired, mock-infected cells. A dashed line indicates the assay limit of detection. (C) moDCs were infected with ZIKV at MOI of 1 in the presence of anti-IFNAR2 blocking antibody. Cells were collected at 48hpi and labeled for ZIKV E protein, while release of infectious virus into the supernatants was determined by FFA. Values for each individual donor are shown with the mean +/- SD (n = 4 donors). A dashed indicates no change relative to infection in the absence of anti-IFNAR2 blocking antibody. (D) RNA was harvested from cells treated the same as for cytokine analysis and IFNB1 mRNA expression was determined by qRT-PCR. Gene expression was normalized to GAPDH transcript levels in each respective sample and represented as the log2 normalized fold increase above donor- and time point-matched untreated cells. Values for each individual donor are shown with the mean (n = 6–8 donors). (E) moDCs were treated with RIG-I agonist (10ng/1e5 cells, 18hrs) or infected with ZIKV PR-2015 (MOI 1 and 10, 48hrs) and analyzed for IFNB1 mRNA expression. Values for each individual donor are shown with the mean (n = 7 donors) (F) IFNβ and IFNα were measured in the supernatant (“Sup”) and whole cell lysate (“WCL”) of moDCs treated the same as in E. Values for each individual donor are shown with the mean (n = 7 donors). Statistical significance (p< 0.05) was determined using a Friedman test with comparisons made to donor-paired, mock-infected cells. (G) Uninfected or ZIKV PR-2015-infected moDCs (MOI 10, 48hpi) were treated with RIG-I agonist (10ng/1e5 cells, 18hrs) and IFNβ and IFNα were measured as in F. The data is shown as the fold-decrease from RIG-I agonist treatment alone with significance (P<0.05) determined using a Mann Whitney Test (n = 4 donors). Error bars represent the mean +/- SD. See also S4 Fig.
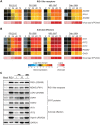
Fig 6. ZIKV infection induces an antiviral state within human DCs.
moDCs were infected with ZIKV PR-2015, P6-1966, MR-1947, or Dak-1984 at MOI of 1 (n = 6–8 donors). Cells were collected at indicated hours post-infection and antiviral gene expression was determined by qRT-PCR. Gene expression was normalized to GAPDH transcript levels in each respective sample and represented as the averaged log2 normalized fold increase above donor and time-point matched uninfected cells. The averaged log10 normalized levels of infectious virus (FFU/mL) at each time point is depicted beneath the gene expression heat map. (A) RLR gene expression. (B) Antiviral effector gene expression. (C) moDCs were left untreated (“Mock”), treated with RIG-I agonist (10ng/1e5 cells), or infected with ZIKV PR-2015 (MOIs of 1 and 10) or MR-1947 (MOI 1). After 18hrs of agonist treatment or at 48hpi with ZIKV, whole-cell lysates were collected for western blot analysis of host antiviral effector protein expression. Western blots are shown for a single donor and are representative of data obtained from two donors. See also S5 Fig.
Fig 7. Innate immune signaling restricts ZIKV viral replication within human DCs.
(A) moDCs were infected with PR-2015, P6-1966, MR-1947, or Dak-1984 at MOI of 1 (n = 4 donors). After viral attachment and entry at 1hpi, cells were treated with RIG-I agonist (10ng/1e5 cells), human IFNβ (100 IU/mL), or left untreated. (B) Supernatants were collected at 48hpi and assessed for infectious virus release by FFA. Values for each individual donor are shown with the mean +/- SD. Statistical significance (p< 0.05) was determined using a Friedman test with comparisons made to donor-paired, untreated, ZIKV-infected cells. The assay limit of detection is indicated with a dashed line.
Fig 8. ZIKV antagonizes type I IFN signaling.
(A, B) A549 cells were infected with PR-2015, P6-1966, MR-1947, or Dak-1984 at MOIs of 0.1 and 1. At 48hpi, cells were pulse treated with 1000 IU/mL of recombinant human IFNβ for 30 minutes and whole-cell lysates were collected for western blot analysis of phospho-STAT1 (Tyr701), phospho-STAT2 (Tyr689), STAT1, STAT2, and GAPDH. Representative blots are shown from one of two independent experiments. Quantitation is shown below the representative blots, where intensity values are represented as the ratio of pSTAT:total STAT protein. (C) moDCs were infected with PR-2015 (MOI 10) and STAT1 and STAT2 signaling was assessed as in A and B. Data is representative of three donors from two independent experiments. Quantitation is shown to the right of the representative blots, where intensity values are represented as the ratio of pSTAT:total STAT protein.
Similar articles
- STAT5: a Target of Antagonism by Neurotropic Flaviviruses.
Zimmerman MG, Bowen JR, McDonald CE, Young E, Baric RS, Pulendran B, Suthar MS. Zimmerman MG, et al. J Virol. 2019 Nov 13;93(23):e00665-19. doi: 10.1128/JVI.00665-19. Print 2019 Dec 1. J Virol. 2019. PMID: 31534033 Free PMC article. - Comparative Analysis of African and Asian Lineage-Derived Zika Virus Strains Reveals Differences in Activation of and Sensitivity to Antiviral Innate Immunity.
Esser-Nobis K, Aarreberg LD, Roby JA, Fairgrieve MR, Green R, Gale M Jr. Esser-Nobis K, et al. J Virol. 2019 Jun 14;93(13):e00640-19. doi: 10.1128/JVI.00640-19. Print 2019 Jul 1. J Virol. 2019. PMID: 31019057 Free PMC article. - West Nile Virus Infection Blocks Inflammatory Response and T Cell Costimulatory Capacity of Human Monocyte-Derived Dendritic Cells.
Zimmerman MG, Bowen JR, McDonald CE, Pulendran B, Suthar MS. Zimmerman MG, et al. J Virol. 2019 Nov 13;93(23):e00664-19. doi: 10.1128/JVI.00664-19. Print 2019 Dec 1. J Virol. 2019. PMID: 31534040 Free PMC article. - Evasion of Innate and Intrinsic Antiviral Pathways by the Zika Virus.
Serman TM, Gack MU. Serman TM, et al. Viruses. 2019 Oct 22;11(10):970. doi: 10.3390/v11100970. Viruses. 2019. PMID: 31652496 Free PMC article. Review. - Functional RNA during Zika virus infection.
Göertz GP, Abbo SR, Fros JJ, Pijlman GP. Göertz GP, et al. Virus Res. 2018 Aug 2;254:41-53. doi: 10.1016/j.virusres.2017.08.015. Epub 2017 Aug 31. Virus Res. 2018. PMID: 28864425 Review.
Cited by
- Zika Virus Transmission Through Blood Tissue Barriers.
Khaiboullina SF, Ribeiro FM, Uppal T, Martynova EV, Rizvanov AA, Verma SC. Khaiboullina SF, et al. Front Microbiol. 2019 Jul 4;10:1465. doi: 10.3389/fmicb.2019.01465. eCollection 2019. Front Microbiol. 2019. PMID: 31333605 Free PMC article. Review. - Zika virus encephalitis in immunocompetent mice is dominated by innate immune cells and does not require T or B cells.
Hayashida E, Ling ZL, Ashhurst TM, Viengkhou B, Jung SR, Songkhunawej P, West PK, King NJC, Hofer MJ. Hayashida E, et al. J Neuroinflammation. 2019 Sep 11;16(1):177. doi: 10.1186/s12974-019-1566-5. J Neuroinflammation. 2019. PMID: 31511023 Free PMC article. - Asian and African lineage Zika viruses show differential replication and innate immune responses in human dendritic cells and macrophages.
Österlund P, Jiang M, Westenius V, Kuivanen S, Järvi R, Kakkola L, Lundberg R, Melén K, Korva M, Avšič-Županc T, Vapalahti O, Julkunen I. Österlund P, et al. Sci Rep. 2019 Oct 31;9(1):15710. doi: 10.1038/s41598-019-52307-1. Sci Rep. 2019. PMID: 31673117 Free PMC article. - Immune Evasion Strategies Used by Zika Virus to Infect the Fetal Eye and Brain.
Nelson BR, Roby JA, Dobyns WB, Rajagopal L, Gale M Jr, Adams Waldorf KM. Nelson BR, et al. Viral Immunol. 2020 Jan/Feb;33(1):22-37. doi: 10.1089/vim.2019.0082. Epub 2019 Nov 5. Viral Immunol. 2020. PMID: 31687902 Free PMC article. Review. - Flaviviruses: Innate Immunity, Inflammasome Activation, Inflammatory Cell Death, and Cytokines.
Pan Y, Cai W, Cheng A, Wang M, Yin Z, Jia R. Pan Y, et al. Front Immunol. 2022 Jan 28;13:829433. doi: 10.3389/fimmu.2022.829433. eCollection 2022. Front Immunol. 2022. PMID: 35154151 Free PMC article. Review.
References
- Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jaaskelainen AJ, et al. (2016) Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N Engl J Med. - PubMed
MeSH terms
Substances
Grants and funding
- R37 AI048638/AI/NIAID NIH HHS/United States
- R38 AI140299/AI/NIAID NIH HHS/United States
- U19 AI057266/AI/NIAID NIH HHS/United States
- R37 DK057665/DK/NIDDK NIH HHS/United States
- U19 AI110819/AI/NIAID NIH HHS/United States
- U19 AI090023/AI/NIAID NIH HHS/United States
- U19 AI083019/AI/NIAID NIH HHS/United States
- R56 AI110516/AI/NIAID NIH HHS/United States
- P51 OD011132/OD/NIH HHS/United States
- R21 AI113485/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous