Seawater acidification induced immune function changes of haemocytes in Mytilus edulis: a comparative study of CO2 and HCl enrichment - PubMed (original) (raw)
Seawater acidification induced immune function changes of haemocytes in Mytilus edulis: a comparative study of CO2 and HCl enrichment
Tianli Sun et al. Sci Rep. 2017.
Abstract
The present study was performed to evaluate the effects of CO2- or HCl-induced seawater acidification (pH 7.7 or 7.1; control: pH 8.1) on haemocytes of Mytilus edulis, and the changes in the structure and immune function were investigated during a 21-day experiment. The results demonstrated that seawater acidification had little effect on the cellular mortality and granulocyte proportion but damaged the granulocyte ultrastructure. Phagocytosis of haemocytes was also significantly inhibited in a clearly concentration-dependent manner, demonstrating that the immune function was affected. Moreover, ROS production was significantly induced in both CO2 and HCl treatments, and four antioxidant components, GSH, GST, GR and GPx, had active responses to the acidification stress. Comparatively, CO2 had more severe destructive effects on haemocytes than HCl at the same pH level, indicating that CO2 stressed cells in other ways beyond the increasing H+ concentration. One possible explanation was that seawater acidification induced ROS overproduction, which damaged the ultrastructure of haemocytes and decreased phagocytosis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Figure 1. Representative TEM micrographs of observed histopathological alterations in the granulocyte of M. edulis.
(a) Normal structure of a granulocyte of a mussel, with dense and boundary clear lysosomes (ly), complete and smooth cytomembranes (cy) and karyothecas (ka), and chromatin evenly distributed in the nucleus (n). (b) Cytoplasmic vacuolation (cv), with many lysosomes having lost their contents. (c) Swollen cytomembrane (cys) and swollen karyotheca (kas), with obvious membrane separations. Severe swelling causes breakages. (d) A seriously injured granulocyte, with a fuzzy boundary of lysosomes (lysosomes dissolved, lyd), chromatin condensation (cc) and extensively swollen cytomembrane (cys).
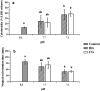
Figure 2. Structural damaging effects of seawater acidification induced structural changes in haemocytes exposed to seawater acidification (mean ± SEM, n = 5).
Note: Different lower case letters indicated the significant difference between the treated groups with the control group at the P < 0.05 level. (a) Acidification caused membrane damage in haemocytes detected by LDH release assay; (b) Acidification caused lysosomal membrane stability damage in haemocytes detected by NRRT assay.
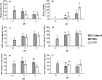
Figure 3. The functional effects of seawater acidification in haemocytes exposed to HG or CG induced seawater acidification at different pH values compared to the control group (mean ± SEM, n = 5).
Different lower case letters indicate significant differences between haemocyte treatments (P < 0.05, ANOVA). (a) Phagocytosis levels of haemocytes; (b) Percent differences in production of ROS in haemocytes; (c) The concentration of GSH in haemocytes; (d) GST activity changes in haemocytes; (e) GR activity changes in haemocytes; (f) GPx activity changes in haemocytes.
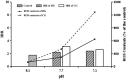
Figure 4. Changes of antioxidant system (IBR consolidation) and ROS concentration in each pH level on 21 d in the HCl adjustment and the CO2 enrichment groups
.
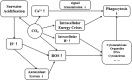
Figure 5. The conjectured pathway of how seawater acidification acts on the structure and immune function of haemocytes of M. edulis
.
Similar articles
- Title: CO2 and HCl-induced seawater acidification impair the ingestion and digestion of blue mussel Mytilus edulis.
Xu M, Sun T, Tang X, Lu K, Jiang Y, Cao S, Wang Y. Xu M, et al. Chemosphere. 2020 Feb;240:124821. doi: 10.1016/j.chemosphere.2019.124821. Epub 2019 Sep 9. Chemosphere. 2020. PMID: 31546185 - Comparative studies on the effects of seawater acidification caused by CO₂ and HCl enrichment on physiological changes in Mytilus edulis.
Sun T, Tang X, Zhou B, Wang Y. Sun T, et al. Chemosphere. 2016 Feb;144:2368-76. doi: 10.1016/j.chemosphere.2015.10.117. Epub 2015 Nov 22. Chemosphere. 2016. PMID: 26610296 - BDE-47 exposure changed the immune function of haemocytes in Mytilus edulis: An explanation based on ROS-mediated pathway.
Jiang Y, Tang X, Sun T, Wang Y. Jiang Y, et al. Aquat Toxicol. 2017 Jan;182:58-66. doi: 10.1016/j.aquatox.2016.11.010. Epub 2016 Nov 11. Aquat Toxicol. 2017. PMID: 27871004 - The ROS-mediated pathway coupled with the MAPK-p38 signalling pathway and antioxidant system plays roles in the responses of Mytilus edulis haemocytes induced by BDE-47.
Jiang Y, Tang X, Zhou B, Sun T, Chen H, Zhao X, Wang Y. Jiang Y, et al. Aquat Toxicol. 2017 Jun;187:55-63. doi: 10.1016/j.aquatox.2017.03.011. Epub 2017 Mar 18. Aquat Toxicol. 2017. PMID: 28371659 - Bioremediation of waste under ocean acidification: Reviewing the role of Mytilus edulis.
Broszeit S, Hattam C, Beaumont N. Broszeit S, et al. Mar Pollut Bull. 2016 Feb 15;103(1-2):5-14. doi: 10.1016/j.marpolbul.2015.12.040. Epub 2016 Jan 8. Mar Pollut Bull. 2016. PMID: 26778338 Review.
Cited by
- Proteomic and Transcriptomic Responses Enable Clams to Correct the pH of Calcifying Fluids and Sustain Biomineralization in Acidified Environments.
Schwaner C, Farhat S, Haley J, Pales Espinosa E, Allam B. Schwaner C, et al. Int J Mol Sci. 2022 Dec 16;23(24):16066. doi: 10.3390/ijms232416066. Int J Mol Sci. 2022. PMID: 36555707 Free PMC article. - Nutrient Alteration Drives the Impacts of Seawater Acidification on the Bloom-Forming Dinoflagellate Karenia mikimotoi.
Liu Q, Wang Y, Li Y, Li Y, Wang Y, Zhou B, Zhou Z. Liu Q, et al. Front Plant Sci. 2021 Oct 21;12:739159. doi: 10.3389/fpls.2021.739159. eCollection 2021. Front Plant Sci. 2021. PMID: 34751224 Free PMC article. - The extensive transgenerational transcriptomic effects of ocean acidification on the olfactory epithelium of a marine fish are associated with a better viral resistance.
Cohen-Rengifo M, Danion M, Gonzalez AA, Bégout ML, Cormier A, Noël C, Cabon J, Vitré T, Mark FC, Mazurais D. Cohen-Rengifo M, et al. BMC Genomics. 2022 Jun 17;23(1):448. doi: 10.1186/s12864-022-08647-w. BMC Genomics. 2022. PMID: 35710351 Free PMC article. - The Bloom-Forming Dinoflagellate Karenia mikimotoi Adopts Different Growth Modes When Exposed to Short or Long Period of Seawater Acidification.
Li Y, Zhou Z, Li Y, Wang Y, Xu M, Zhou B, Lu K, Wang Y. Li Y, et al. Toxins (Basel). 2021 Sep 8;13(9):629. doi: 10.3390/toxins13090629. Toxins (Basel). 2021. PMID: 34564633 Free PMC article. - Molecular Features Associated with Resilience to Ocean Acidification in the Northern Quahog, Mercenaria mercenaria.
Schwaner C, Farhat S, Barbosa M, Boutet I, Tanguy A, Pales Espinosa E, Allam B. Schwaner C, et al. Mar Biotechnol (NY). 2023 Feb;25(1):83-99. doi: 10.1007/s10126-022-10183-3. Epub 2022 Nov 22. Mar Biotechnol (NY). 2023. PMID: 36417051
References
- Dupont S. & Pörtner H. O. Marine science: Get ready for ocean acidification. Nature 498, 429–429 (2013). - PubMed
- Caldeira K. & Wickett M. E. Oceanography: anthropogenic carbon and ocean pH. Nature 425, 365–365 (2003). - PubMed
- Reguera D. F., Riba I., Forja J. M. & DelValls T. Á. An integrated approach to determine sediment quality in areas above CO2 injection and storage in agreement with the requirements of the international conventions on the protection of the marine environment. Ecotoxicology 18, 1123–1129 (2009). - PubMed
- Bellerby R. G. J. & Golmen L. G. Report from Workshop 2, Novel Technologies (2013).
- Kroeker K. J., Kordas R. L., Crim R. N. & Singh G. G. Meta‐analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. lett. 13, 1419–1434 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials