Discovery of Mieap-regulated mitochondrial quality control as a new function of tumor suppressor p53 - PubMed (original) (raw)
Review
. 2017 May;108(5):809-817.
doi: 10.1111/cas.13208. Epub 2017 May 5.
Affiliations
- PMID: 28222492
- PMCID: PMC5448595
- DOI: 10.1111/cas.13208
Review
Discovery of Mieap-regulated mitochondrial quality control as a new function of tumor suppressor p53
Yasuyuki Nakamura et al. Cancer Sci. 2017 May.
Abstract
The tumor suppressor p53 gene is frequently mutated in human cancers, and the p53 protein suppresses cancer. However, the mechanism behind the p53-mediated tumor suppression is still unclear. Recently, the mitochondria-eating protein (Mieap) was identified as a p53-inducible protein. Mieap induces the accumulation of lysosomal proteins within mitochondria (Mieap-induced accumulation of lysosome-like organelles within mitochondria, or MALM) in response to mitochondrial damage, and eliminates the oxidized mitochondrial proteins to repair unhealthy mitochondria. Furthermore, Mieap also induces vacuole-like structures (Mieap-induced vacuole, or MIV) to eat and degrade unhealthy mitochondria. Therefore, Mieap controls mitochondrial quality by repairing or eliminating unhealthy mitochondria by MALM or MIV, respectively. This mechanism is not mediated by canonical autophagy. Mieap-deficient ApcMin/+ mice show strikingly high rates of intestinal tumor development as well as advanced-grade adenomas and adenocarcinomas. The p53/Mieap/BCL2 interacting protein 3 mitochondrial quality control pathway is frequently inactivated in human colorectal cancers. Defects in Mieap-regulated mitochondrial quality control lead to accumulation of unhealthy mitochondria in cancer cells. Cancer-specific unhealthy mitochondria could contribute to cancer development and aggressiveness through mitochondrial reactive oxygen species and altered metabolism. Mieap-regulated mitochondrial quality control is a newly discovered function of p53 that plays a critical role in tumor suppression.
Keywords: Autophagy; cancer metabolism; mitochondrial quality control; reactive oxygen species; tumor suppressor p53.
© 2017 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures
Figure 1
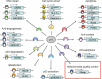
Tumor suppressor p53 regulates a large number of functions through transcriptional activation of its target genes as a transcription factor. Apoptosis, cell cycle arrest,
DNA
repair, and anti‐angiogenesis are believed to be the core functions for p53‐mediated tumor suppression. Mieap is a p53‐target gene, and the function is involved in mitochondrial quality control.
Figure 2
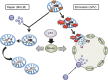
Mitochondria‐eating protein (Mieap)‐regulated mitochondrial quality control. In response to mitochondrial damage, p53‐activated Mieap induces accumulation of lysosomal proteins within mitochondria to eliminate the oxidized mitochondrial proteins (
MALM
). The
MALM
repairs unhealthy mitochondria. Mieap also induces large vacuole‐like structures (
MIV
) to eat and degrade very dangerous and unhealthy mitochondria producing high levels of reactive oxygen species (
ROS
). Therefore, p53/Mieap maintains mitochondrial integrity by repairing or eliminating unhealthy mitochondria by
MALM
or
MIV
, respectively. Lyso, lysosome.
Figure 3
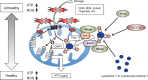
Hypothetical model for the mitochondria‐eating protein (Mieap)‐induced accumulation of lysosome‐like organelles within mitochondria (
MALM
) mechanism. Unhealthy mitochondria produce high level of reactive oxygen species (
ROS
). Mitochondrial
ROS
induces the interaction of Mieap and two mitochondrial outer‐membrane proteins,
BCL
2 interacting protein 3 (
BNIP
- and
NIX
. The interaction of Mieap,
BNIP
3, and
NIX
leads to the formation of a pore, through which lysosomal proteins or lysosome‐like organelles accumulate within mitochondria. 14‐3‐3γ interacts with Mieap in cytosol, and translocates into the mitochondria, which mediates the elimination of the oxidized mitochondrial proteins in the mitochondria. Therefore,
MALM
maintains the healthy status of the mitochondria, indicating an increase of
ATP
synthesis activity and a decrease of
ROS
generation.
CMA
, chaperone‐mediated autophagy.
Figure 4
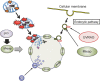
Hypothetical model for the mitochondria‐eating protein (Mieap)‐induced vacuole (
MIV
) mechanism. Severely damaged and dangerous mitochondria produce very high levels of reactive oxygen species (
ROS
), which are extremely toxic to the cell. These
ROS
induce the generation of
MIV
s. The
MIV
s uptake and degrade the dangerous mitochondria to maintain cellular homeostasis.
UV
radiation resistance associated gene (
UVRAG
) protein mediates the
MIV
formation by interacting with Mieap. The endocytic pathway also plays a critical role in
MIV
formation. Lyso, lysosome.
Figure 5
Interplay between mitochondria‐eating protein (Mieap)‐induced accumulation of lysosome‐like organelles within mitochondria (
MALM
) and Mieap‐induced vacuoles (
MIV
): repair? Or elimination? Four cancer cell lines derived from A549 (lung cancer cell line), control,
NIX
‐knockdown (
KD
), p53‐
KD
, and Mieap‐
KD
, were irradiated by ionizing radiation (
IR
). 3 days after
IR
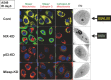
, immunostaining experiments were carried out with anti‐Mieap antibody (Mieap), anti‐cathepsin D antibody (Cathepsin‐D), and DsRed‐mito (Mitochondria).
MALM
occurred in the control cells, in which the colocalization of Mieap, lysosome, and mitochondria is observed (yellow signals). When
MALM
is inhibited by
NIX
‐
KD
,
MIV
s appear, eat, and degrade the mitochondria in the
NIX
‐
KD
cells. Both
MALM
and
MIV
are cancelled in the p53‐
KD
and Mieap‐
KD
cells. Therefore,
MALM
and
MIV
are closely related to each other when making a decision about repair or elimination of unhealthy mitochondria. Both
MALM
and
MIV
are strictly regulated by the p53/Mieap pathway. Reproduced from Kitamura et al.37 with permission from.
EM
, electron microscopy.
Figure 6
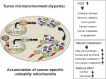
Cancer‐specific unhealthy mitochondria in the hypoxic tumor microenvironment function as a driving force for cancer development and progression. In human cancer, mitochondria‐eating protein (Mieap)‐regulated mitochondrial quality control is frequently inactivated by p53 mutations and/or Mieap/
BCL
2 interacting protein 3 (
BNIP
- promoter methylation. This leads to the accumulation of cancer specific unhealthy mitochondria in the hypoxic tumor microenvironment. Cancer‐specific unhealthy mitochondria produce high levels of
ROS
. Mitochondrial
ROS
induce oxidative damage and genomic instability, and promote tumor growth, cancer invasion, metastasis, and tumor angiogenesis. Cancer specific unhealthy mitochondria also causes altered metabolism including activation of fatty acid synthesis, nucleic acid synthesis, glutamate metabolism, and one carbon metabolism, defective oxidative phosphorylation, and upregulation of glycolysis. Therefore, mitochondrial reactive oxygen species (
ROS
) and altered metabolism caused by cancer‐specific unhealthy mitochondria could greatly contribute to cancer development and progression.
Similar articles
- Mieap, a p53-inducible protein, controls mitochondrial quality by repairing or eliminating unhealthy mitochondria.
Kitamura N, Nakamura Y, Miyamoto Y, Miyamoto T, Kabu K, Yoshida M, Futamura M, Ichinose S, Arakawa H. Kitamura N, et al. PLoS One. 2011 Jan 17;6(1):e16060. doi: 10.1371/journal.pone.0016060. PLoS One. 2011. PMID: 21264228 Free PMC article. - Possible existence of lysosome-like organella within mitochondria and its role in mitochondrial quality control.
Miyamoto Y, Kitamura N, Nakamura Y, Futamura M, Miyamoto T, Yoshida M, Ono M, Ichinose S, Arakawa H. Miyamoto Y, et al. PLoS One. 2011 Jan 17;6(1):e16054. doi: 10.1371/journal.pone.0016054. PLoS One. 2011. PMID: 21264221 Free PMC article. - Mieap-regulated mitochondrial quality control is frequently inactivated in human colorectal cancer.
Kamino H, Nakamura Y, Tsuneki M, Sano H, Miyamoto Y, Kitamura N, Futamura M, Kanai Y, Taniguchi H, Shida D, Kanemitsu Y, Moriya Y, Yoshida K, Arakawa H. Kamino H, et al. Oncogenesis. 2016 Jan 4;4(1):e181. doi: 10.1038/oncsis.2015.43. Oncogenesis. 2016. PMID: 26727575 Free PMC article. - p53 and mitochondrial dysfunction: novel insight of neurodegenerative diseases.
Dai CQ, Luo TT, Luo SC, Wang JQ, Wang SM, Bai YH, Yang YL, Wang YY. Dai CQ, et al. J Bioenerg Biomembr. 2016 Aug;48(4):337-47. doi: 10.1007/s10863-016-9669-5. Epub 2016 Jul 15. J Bioenerg Biomembr. 2016. PMID: 27422544 Free PMC article. Review. - The role of tumor suppressor p53 in metabolism and energy regulation, and its implication in cancer and lifestyle-related diseases.
Hashimoto N, Nagano H, Tanaka T. Hashimoto N, et al. Endocr J. 2019 Jun 28;66(6):485-496. doi: 10.1507/endocrj.EJ18-0565. Epub 2019 May 18. Endocr J. 2019. PMID: 31105124 Review.
Cited by
- Comparison between SPATA18 and P53 Gene Expressions in The Sperm Cells Obtained from Normospermic and Asthenospermic Samples: A Case-Control Study.
Panahi A, Mirza Ahmadi S, Asaadi Tehrani G. Panahi A, et al. Int J Fertil Steril. 2022 Apr;16(2):122-127. doi: 10.22074/IJFS.2021.138190.1029. Epub 2022 May 8. Int J Fertil Steril. 2022. PMID: 35639655 Free PMC article. - MIEAP and ATG5 are tumor suppressors in a mouse model of BRAFV600E-positive thyroid cancer.
Hamada K, Kurashige T, Shimamura M, Arakawa H, Nakamura Y, Nagayama Y. Hamada K, et al. Front Endocrinol (Lausanne). 2022 Sep 15;13:932754. doi: 10.3389/fendo.2022.932754. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36187114 Free PMC article. - Mitochondria: Endosymbiont bacteria DNA sequence as a target against cancer.
Nagase H, Watanabe T, Koshikawa N, Yamamoto S, Takenaga K, Lin J. Nagase H, et al. Cancer Sci. 2021 Dec;112(12):4834-4843. doi: 10.1111/cas.15143. Epub 2021 Oct 4. Cancer Sci. 2021. PMID: 34533888 Free PMC article. Review. - Characterization of metabolic reprogramming by metabolomics in the oncocytic thyroid cancer cell line XTC.UC1.
Kurashige T, Shimamura M, Hamada K, Matsuse M, Mitsutake N, Nagayama Y. Kurashige T, et al. Sci Rep. 2023 Jan 4;13(1):149. doi: 10.1038/s41598-023-27461-2. Sci Rep. 2023. PMID: 36599897 Free PMC article. - p53 Gene (NY-CO-13) Levels in Patients with Chronic Myeloid Leukemia: The Role of Imatinib and Nilotinib.
Al-Kuraishy HM, Al-Gareeb AI, Al-Buhadilly AK. Al-Kuraishy HM, et al. Diseases. 2018 Jan 25;6(1):13. doi: 10.3390/diseases6010013. Diseases. 2018. PMID: 29370077 Free PMC article.
References
- Lane DP, Crawford LV. T antigen is bound to a host protein in SV40‐transformed cells. Nature 1979; 278: 261–3. - PubMed
- Linzer DI, Levine AJ. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40‐transformed cells and uninfected embryonal carcinoma cells. Cell 1979; 17: 43–52. - PubMed
- Melero JA, Stitt DT, Mangel WF, Carroll RB. Identification of new polypeptide species [48‐55K] immunoprecipitated by antiserum to purified large T antigen and present in SV40‐infected and transformed cells. Virology 1979; 93: 466–80. - PubMed
- Smith AE, Smith R, Paucha E. Characterization of different tumor antigens present in cells transformed by simian virus 40. Cell 1979; 18: 335–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous