Dynasore impairs VEGFR2 signalling in an endocytosis-independent manner - PubMed (original) (raw)
Dynasore impairs VEGFR2 signalling in an endocytosis-independent manner
Dimitris Basagiannis et al. Sci Rep. 2017.
Abstract
VEGFR2 is a critical angiogenic receptor playing a key role in vascular homeostasis. Upon activation by VEGF, VEGFR2 becomes endocytosed. Internalisation of VEGFR2 is facilitated, in part, through clathrin mediated endocytosis (CME), the role of which in VEGFR2 function is debated. Here, we confirm the contribution of CME in VEGFR2 uptake. However, curiously, we find that different approaches of inhibition of CME exert contradictory effects on VEGF signalling; knockdown of clathrin, or of dynamin, or overexpression of dynamin K44A, do not affect VEGF-induced phosphorylation of ERK1/2, while dynasore causes strong inhibition. We resolve this discrepancy by showing that although dynasore inhibits CME of VEGFR2, its inhibitory action in ERK1/2 phosphorylation is not related to attenuation of VEGFR2 endocytosis; it is rather due to an off-target effect of the drug. Dynasore inhibits VEGF-induced calcium release, a signalling event that lies upstream of ERK1/2, which implies that this effect could be responsible, at least in part, for the inhibitory action of the drug on VEGF-to-ERK1/2 signalling. These results raise caution that although dynasore is specific in inhibiting clathrin- and dynamin-mediated endocytosis, it may also exert off-target effects on signalling molecules, hence influencing the interpretation of the role of endocytosis in signalling.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
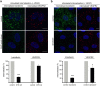
Figure 1. Clathrin-mediated endocytosis of VEGFR2 is consistently inhibited by either knockdown of clathrin or by treatment with dynasore.
(a) Serum starved (2 h) HUVECs, that were transfected with siRNAs against CHC, were incubated with a mouse anti-VEGFR2 extracellular domain antibody at 4 °C, transferred to 37 °C and stimulated with VEGF, in the presence of FITC-transferrin. Prior to fixation, membrane bound antibodies and transferrin were removed by acid wash and the internalised receptor was revealed by secondary fluorescent antibodies, using confocal microscopy. Nuclei are shown in blue (10 μm scale bars). Quantification of VEGFR2 internalisation and transferrin is shown at the bottom of the immunofluorescence images (20 cells from 3 independent experiments were analysed, mean ± S.D., ***P < 0.001, t-test). (b) 2 h serum starved HUVECs were incubated with a mouse anti-VEGFR2 extracellular domain antibody at 4 °C, treated with vehicle (control) or dynasore at 4 °C for 30 min, transferred to 37 °C and processed as described in “a”. Nuclei are shown in blue (10 μm scale bars). Quantification of VEGFR2 internalisation and transferrin is shown at the bottom of the immunofluorescence images (20 cells from 3 independent experiments were analysed, mean ± S.D., *P < 0.05, ***P < 0.001, t-test).
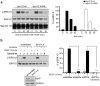
Figure 2. Although knockdown of CHC does not affect VEGF-to-ERK1/2 signalling, dynasore causes a strong inhibitory effect.
(a) HUVECs transfected with siRNAs against clathrin heavy chain were stimulated with VEGF for various time points, lysed and subjected to immunoblotting analysis using antibodies against the phosphorylated or total ERK1/2. Quantification of the effect of clathrin knockdown on the VEGF-induced phosphorylation of ERK1/2 is shown on the right of the immunoblots. The effect is considered non-significant (n = 3, mean ± S.E.M., ANOVA followed by Dunnett’s analysis). (b) Serum starved (2 h) HUVECs were treated with vehicle or dynasore (100 μM), stimulated with VEGF for various time points, lysed and subjected to immunoblotting analysis using antibodies against the phosphorylated or total ERK1/2. Quantification of the effect of dynasore treatment on the VEGF-induced phosphorylation of ERK1/2 is shown on the right of the immunoblots (n = 3, mean ± S.E.M., P < 0.001, ANOVA followed by Dunnett’s analysis).
Figure 3. Dynasore-mediated inhibition of VEGF-to-ERK1/2 signalling is independent of dynamin.
(a) HUVECs transduced with lentiviral vectors encoding dynamin (1 and 2) or dynamin K44A (1 and 2) were stimulated with VEGF for various time points, lysed and subjected to immunoblotting analysis using antibodies against the phosphorylated or total ERK1/2. Quantification of the effect of dynamin inhibition on the VEGF-induced phosphorylation of ERK1/2 is shown on the right of the immunoblots. The effect is considered non-significant (n = 3, mean ± S.D., ANOVA followed by Dunnett’s analysis). (b) Dynamin2 siRNAs treated HUVECs were serum starved for 2 h, incubated with 100 μM dynasore for 30 min and stimulated with VEGF for 10 min, lysed and processed for immunoblotting analysis using antibodies against the phosphorylated and total forms of ERK1/2. Immunoblots are representative of 3 independent experiments. Quantification is shown on the right of the immunoblots (n = 3, mean ± S.D., ***P < 0.001, ANOVA followed by Dunnett’s analysis). The efficiency of dynamin2 knockdown was assessed by semi-quantitative RT-PCR (see agarose gel at the bottom of b), as well as by immunofluorescence microscopy analysis of transferrin uptake (see S1b).
Figure 4. Dynasore inhibits signalling and endocytosis with different efficacies.
(a) Immunoblotting analysis of the dose-dependent effect of dynasore on the VEGF-induced phosphorylation of ERK1/2 in HUVECs. Quantification of the phospho-ERK1/2 zones is shown on the right of the immunoblots (n = 3, mean ± S.D., **P < 0.05, ***P < 0.001, t-test). (b) Analysis of the dose-dependent effect of dynasore on CME. HUVECs were pre-treated (30 min) with different concentrations of dynasore and incubated with FITC-transferrin for 15 min, acid-washed, fixed and processed for immunofluorescence microscopy analysis (15 microscopy fields from 3 independent experiments were analysed, mean ± S.D., *P < 0.05, **P < 0.005, ***P < 0.001, t-test). (c) Dose response comparison of the inhibitory effect of dynasore on transferrin internalisation versus inhibition of VEGF-to-ERK1/2 phosphorylation. Graph bars express the % of internalised transferrin, or ERK1/2 phosphorylation, of dynasore treated cells relatively to vehicle treated cells.
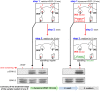
Figure 5. Dynasore-mediated inhibition of VEGF-to-ERK1/2 signalling is independent of DDE (“uncoupling experiment”).
In step 1, HUVECs were treated with vehicle (left side) or dynasore (right side) and stimulated with VEGF (in the presence of vehicle or drug), for 10 min. The scheme (step 1, right side) illustrates that dynasore blocks both dynamin-dependent endocytosis (indicated in the scheme as “DDE blocked”) and the off-targets (indicated in the scheme as “off-targets are inhibited by dynasore”), while dynamin-independent endocytosis (DIE) remains unaffected. Then, the cells were either lysed and subjected to immunoblotting analysis, to test the effect of dynasore on ERK1/2 phosphorylation when the drug is continuously present (see lanes depicted by the long vertical arrow, lanes 2 and 4), or were acid washed (step 2), to remove membrane bound VEGF and dynasore (the effectiveness of acid wash treatment to remove VEGF from plasma membrane is shown in Fig. S4a), and processed to the next step. In step 3, cells were incubated in serum-free medium, for 10 min, to allow full recovery from dynasore, thereby releasing possible off-target effects of the drug (indicated in the scheme as “off-targets: released”, see step 3, right). As there is no ligand in this phase, DDE cannot resume despite removal of the drug (indicated in the scheme as “DDE blocked”). Thus, in lane 5, DDE does not take place throughout the whole experiment, even after removal of the drug (note that this is the main difference with the reversibility experiment shown in Fig. S2a, where VEGF is added after the removal of the drug, which rescues both DDE and the off-targets). Then, the cells were lysed and subjected to immunoblotting analysis using anti-phospho-ERK1/2 antibodies. Since DDE never took place in sample 5 (see above), recovery of the signal in this sample (compare lane 5 with lane 4), upon release of the off-targets, suggests that DDE is not essential for VEGF signalling; thus, the inhibitory effect of dynasore in lane 4 is due to the off-targets. At the bottom of the figure there is a schematic summary of the treatment steps of sample 5. Full-length blots are shown in Fig. S4b.
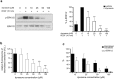
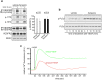
Figure 6. Dynasore inhibits VEGF-induced activation of calcium release.
(a) Dynasore does not affect VEGF-induced phosphorylation of VEGFR2. Vehicle or dynasore treated serum deprived HUVECs were stimulated with VEGF for 5 min, lysed and subjected to immunoblotting analysis using antibodies against phosphorylated VEGFR2 at tyrosine 1175 or 1214. Total phosphorylation of the receptor was revealed by VEGFR2 immunoprecipitation and immunoblotting analysis of the precipitants using anti-phosphotyrosine antibodies. Quantification is shown on the right of the immunoblots (n = 3, mean ± S.D., t-test). (b) Dynasore potentiates phosphorylation of PLCγ. Vehicle or dynasore treated HUVECs were stimulated with VEGF (for the indicated time periods), lysed and analysed by immunoblotting using antibodies against phosphorylated and total PLCγ. (c) Time-course graph of VEGF-induced Ca2+ fluorescence in HUVECs treated with vehicle or dynasore in the presence of the Ca2+ indicator fluo-4 AM. The graph is representative of three independent experiments.
Similar articles
- Chemical Inhibitors of Dynamin Exert Differential Effects in VEGF Signaling.
Basagiannis D, Zografou S, Goula E, Gkeka D, Kolettas E, Christoforidis S. Basagiannis D, et al. Cells. 2021 Apr 23;10(5):997. doi: 10.3390/cells10050997. Cells. 2021. PMID: 33922806 Free PMC article. - VEGF induces signalling and angiogenesis by directing VEGFR2 internalisation through macropinocytosis.
Basagiannis D, Zografou S, Murphy C, Fotsis T, Morbidelli L, Ziche M, Bleck C, Mercer J, Christoforidis S. Basagiannis D, et al. J Cell Sci. 2016 Nov 1;129(21):4091-4104. doi: 10.1242/jcs.188219. Epub 2016 Sep 21. J Cell Sci. 2016. PMID: 27656109 - Dynasore puts a new spin on dynamin: a surprising dual role during vesicle formation.
Nankoe SR, Sever S. Nankoe SR, et al. Trends Cell Biol. 2006 Dec;16(12):607-9. doi: 10.1016/j.tcb.2006.10.004. Epub 2006 Oct 24. Trends Cell Biol. 2006. PMID: 17064900 - Dynasore - not just a dynamin inhibitor.
Preta G, Cronin JG, Sheldon IM. Preta G, et al. Cell Commun Signal. 2015 Apr 10;13:24. doi: 10.1186/s12964-015-0102-1. Cell Commun Signal. 2015. PMID: 25889964 Free PMC article. Review. - VEGF-A-stimulated signalling in endothelial cells via a dual receptor tyrosine kinase system is dependent on co-ordinated trafficking and proteolysis.
Bruns AF, Bao L, Walker JH, Ponnambalam S. Bruns AF, et al. Biochem Soc Trans. 2009 Dec;37(Pt 6):1193-7. doi: 10.1042/BST0371193. Biochem Soc Trans. 2009. PMID: 19909245 Review.
Cited by
- Clathrin-mediated endocytosis regulates fMLP-mediated neutrophil polarization.
Tan X, Luo M, Liu AP. Tan X, et al. Heliyon. 2018 Sep 24;4(9):e00819. doi: 10.1016/j.heliyon.2018.e00819. eCollection 2018 Sep. Heliyon. 2018. PMID: 30263974 Free PMC article. - Shear Stress and Sub-Femtomolar Levels of Ligand Synergize to Activate ALK1 Signaling in Endothelial Cells.
Cheng YW, Anzell AR, Morosky SA, Schwartze TA, Hinck CS, Hinck AP, Roman BL, Davidson LA. Cheng YW, et al. Cells. 2024 Feb 5;13(3):285. doi: 10.3390/cells13030285. Cells. 2024. PMID: 38334677 Free PMC article. - Arf6 Regulates Endocytosis and Angiogenesis by Promoting Filamentous Actin Assembly.
Francis CR, Bell ML, Skripnichuk MM, Kushner EJ. Francis CR, et al. bioRxiv [Preprint]. 2023 Feb 22:2023.02.22.529543. doi: 10.1101/2023.02.22.529543. bioRxiv. 2023. PMID: 36865161 Free PMC article. Updated. Preprint. - Functional Autophagic Flux Regulates AgNP Uptake And The Internalized Nanoparticles Determine Tumor Cell Fate By Temporally Regulating Flux.
Fageria L, Bambroo V, Mathew A, Mukherjee S, Chowdhury R, Pande S. Fageria L, et al. Int J Nanomedicine. 2019 Nov 20;14:9063-9076. doi: 10.2147/IJN.S222211. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31819419 Free PMC article. - Loss of EGF receptor polarity enables homeostatic imbalance in epithelial-cell models.
Carlin CR, Ngalula S. Carlin CR, et al. Mol Biol Cell. 2023 Nov 1;34(12):ar116. doi: 10.1091/mbc.E23-04-0133. Epub 2023 Aug 30. Mol Biol Cell. 2023. PMID: 37647145 Free PMC article.
References
- Miaczynska M., Pelkmans L. & Zerial M. Not just a sink: endosomes in control of signal transduction. Curr Opin Cell Biol 16, 400–406 (2004). - PubMed
- Platta H. W. & Stenmark H. Endocytosis and signaling. Curr Opin Cell Biol 23, 393–403 (2011). - PubMed
- McMahon H. T. & Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 12, 517–533 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous