Distinct functions of dynamin isoforms in tumorigenesis and their potential as therapeutic targets in cancer - PubMed (original) (raw)
Review
Distinct functions of dynamin isoforms in tumorigenesis and their potential as therapeutic targets in cancer
Jianghui Meng. Oncotarget. 2017.
Abstract
Dynamins and their related proteins participate in the regulation of neurotransmission, antigen presentation, receptor internalization, growth factor signalling, nutrient uptake, and pathogen infection. Recently, emerging findings have shown dynamin proteins can also contribute to the genesis of cancer. This up-to-date review herein focuses on the functionality of dynamin in cancer development. Dynamin 1 and 2 both enhance cancer cell proliferation, tumor invasion and metastasis, whereas dynamin 3 has tumor suppression role. Antisense RNAs encoded on the DNA strand opposite a dynamin gene regulate the function of dynamin, and manipulate oncogenes and tumor suppressor genes. Certain dynamin-related proteins are also upregulated in distinct cancer conditions, resulting in apoptotic resistance, cell migration and poor prognosis. Altogether, dynamins are potential biomarkers as well as representing promising novel therapeutic targets for cancer treatment. This study also summarizes the current available dynamin-targeted therapeutics and suggests the potential strategy based on signalling pathways involved, providing important information to aid the future development of novel cancer therapeutics by targeting these dynamin family members.
Keywords: amphiphysin; cancer target; clathrin; endocytosis; tumor.
Conflict of interest statement
CONFLICTS OF INTEREST
The author declares that there is no conflict of interest.
Figures
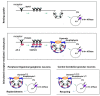
Figure 1. Schematic diagram for resting and stimulated receptor-mediated endocytosis in neurons and differential involvement of dynamin isoforms in stimulated receptor-mediated endocytosis
Using a molecular probe, such as botulinum neurotoxin (BoNT) which cleaves SNARE proteins, to dissect the path of receptor-mediated endocytosis [115], it was revealed that resting uptake by neurons occurs via lipid rafts, acidified compartments and protein acceptors but not dynamin. In contrast, stimulated endocytosis of BoNT by neurons utilises lipid rafts, acidified compartments (Vacuolar-type H+ -ATPase) and dynamin and amphiphysin [4]. In terms of fast recycling of small clear synaptic vesicles, cerebellar granule neurons (CGNs) use predominantly dynamin 1, whereas isoform 2 and, to a lesser extent, isoform 3 to support a less rapid mode of stimulated endocytosis. In contrast, large dense-cored vesicle (LDCV)-releasing trigeminal ganglionic neurons (TGNs) preferentially employ dynamins 2 and 3 and amphiphysin 1 for evoked endocytosis. PM = plasma membrane, V-H+ = Vacuolar-type H+, CCP = clathrin coated pit, CME = clathrin mediated endocytosis.
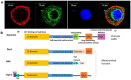
Figure 2. Distinct localization of dynamin isoforms in the peripheral neurons highlights their distinct functional importance
The linear domain arrangement of human dynamin and related proteins. A. Immuno-fluorescence study demonstrated that dynamin 1 and 2 showed distinct distribution pattern in the cultured trigeminal ganglionic neuron. Dynamin 1, green; dynamin 2, red; nuclei, blue. B. Each domain of dynamin protein is indicated as follows: G domain, yellow, is responsible for GTP binding and hydrolysis; middle coiled-coil domain– GTPase Effector Domain (GED) stalk, blue, is responsible for dynamin protein self-assembly, and variant splicing; PH domain (pleckstrin homology), green, is responsible for lipid binding; GED, orange, is responsible for ring assembling and also enhance GTPase activity; PRD (proline rich domain), light purple, interacts with SH3-domain partners, such as amphyphisin; N-terminal MIS (mitochondrial import sequence): dark purple, is responsible for targeting the OPA1 protein; B insert domain (pink) of the Dnm1 guanosine triphosphatase (a Drp) contains a novel motif required for association with the mitochondrial adaptor Mdv1 to the mitochondria.
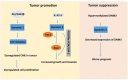
Figure 3. Schematic map for potential targets in dynamin-mediated tumor development whereas dynamin 1 and 2 act as the tumor promotors
In contrast, dynamin 3 has tumor suppressive role. Inhibition or depletion of the Akt/GSK3β signalling pathway will prevent the function of dynamin 1 in lung cancer cells. Moreover, inhibition of dynamin 2 interaction with Vav1 will stop the activation of Rac1, and a method that prevents phosphorylation of PDGFRα-PI3K/SHP-2 will reduce the increased tumor growth and cancer cell invasion in certain types of cancer. Blockage of hypermethylation of DNM3 by some genetic approach or increase of expression of dynamin 3 protein will prevent the tumor development.
Similar articles
- Expression of the endocytic proteins dynamin and amphiphysin in rat gastric enterochromaffin-like cells.
Zanner R, Gratzl M, Prinz C. Zanner R, et al. J Cell Sci. 2004 May 1;117(Pt 11):2369-76. doi: 10.1242/jcs.01091. J Cell Sci. 2004. PMID: 15126636 - Molecular components required for resting and stimulated endocytosis of botulinum neurotoxins by glutamatergic and peptidergic neurons.
Meng J, Wang J, Lawrence GW, Dolly JO. Meng J, et al. FASEB J. 2013 Aug;27(8):3167-80. doi: 10.1096/fj.13-228973. Epub 2013 May 2. FASEB J. 2013. PMID: 23640057 - The role of EHD proteins at the neuronal synapse.
Ioannou MS, Marat AL. Ioannou MS, et al. Sci Signal. 2012 Apr 24;5(221):jc1. doi: 10.1126/scisignal.2002989. Sci Signal. 2012. PMID: 22534130 Review. - Regulatory mechanisms of dynamin-dependent endocytosis.
Takei K, Yoshida Y, Yamada H. Takei K, et al. J Biochem. 2005 Mar;137(3):243-7. doi: 10.1093/jb/mvi052. J Biochem. 2005. PMID: 15809324 Review. - UNC119 inhibits dynamin and dynamin-dependent endocytic processes.
Karim Z, Vepachedu R, Gorska M, Alam R. Karim Z, et al. Cell Signal. 2010 Jan;22(1):128-37. doi: 10.1016/j.cellsig.2009.09.022. Epub 2009 Sep 23. Cell Signal. 2010. PMID: 19781630
Cited by
- CD99 Expression in Glioblastoma Molecular Subtypes and Role in Migration and Invasion.
Cardoso LC, Soares RDS, Laurentino TS, Lerario AM, Marie SKN, Oba-Shinjo SM. Cardoso LC, et al. Int J Mol Sci. 2019 Mar 6;20(5):1137. doi: 10.3390/ijms20051137. Int J Mol Sci. 2019. PMID: 30845661 Free PMC article. - Enriched transcriptome analysis of laser capture microdissected populations of single cells to investigate intracellular heterogeneity in immunostained FFPE sections.
Hammoudeh SM, Hammoudeh AM, Venkatachalam T, Rawat S, Jayakumar MN, Rahmani M, Hamoudi R. Hammoudeh SM, et al. Comput Struct Biotechnol J. 2021 Sep 14;19:5198-5209. doi: 10.1016/j.csbj.2021.09.010. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 34745451 Free PMC article. - Folic Acid Induces Intake-Related Changes in the Mammary Tissue Transcriptome of C57BL/6 Mice.
Burton MA, Antoun E, Penailillo RS, Burdge GC, Lillycrop KA. Burton MA, et al. Nutrients. 2020 Sep 15;12(9):2821. doi: 10.3390/nu12092821. Nutrients. 2020. PMID: 32942660 Free PMC article. - Phenotype versus genotype to optimize cancer dosing in the clinical setting-focus on 5-fluorouracil and tyrosine kinase inhibitors.
Martin JH, Galettis P, Flynn A, Schneider J. Martin JH, et al. Pharmacol Res Perspect. 2024 Apr;12(2):e1182. doi: 10.1002/prp2.1182. Pharmacol Res Perspect. 2024. PMID: 38429945 Free PMC article. Review. - Caveolin-1 and dynamin-2 overexpression is associated with the progression of bladder cancer.
Raja SA, Shah STA, Tariq A, Bibi N, Sughra K, Yousuf A, Khawaja A, Nawaz M, Mehmood A, Khan MJ, Hussain A. Raja SA, et al. Oncol Lett. 2019 Jul;18(1):219-226. doi: 10.3892/ol.2019.10310. Epub 2019 May 3. Oncol Lett. 2019. PMID: 31289491 Free PMC article.
References
- Bauerfeind R, Takei K, De Camilli P. Amphiphysin I is associated with coated endocytic intermediates and undergoes stimulation-dependent dephosphorylation in nerve terminals. J Biol Chem. 1997;272:30984–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources