Dysregulation of RNA Binding Protein Aggregation in Neurodegenerative Disorders - PubMed (original) (raw)
Review
Dysregulation of RNA Binding Protein Aggregation in Neurodegenerative Disorders
Brandon Maziuk et al. Front Mol Neurosci. 2017.
Abstract
The unique biology of RNA binding proteins is altering our view of the genesis of protein misfolding diseases. These proteins use aggregation of low complexity domains (LCDs) as a means to regulate the localization and utilization of RNA by forming RNA granules, such as stress granules, transport granules and P-bodies. The reliance on reversible aggregation as a mechanism for biological regulation renders this family of proteins highly vulnerable to promoting diseases of protein misfolding. Mutations in RNA binding proteins are associated with many neurodegenerative disorders, such as amyotrophic lateral sclerosis (ALS) and frontotemporal lobar dementia (FTLD). The biology of RNA binding proteins also extends to microtubule associated protein tau. Tau is normally an axonal protein, but in stress it translocates to the somatodendritic arbor where it takes on a new function promoting formation of stress granules. The interaction of tau with stress granules also promotes tau aggregation, accelerating formation of the tau pathology that we associate with diseases such as Alzheimer's disease (AD).
Keywords: RNA Translation; RNA binding proteins; RNA metabolism; stress response; tau aggregation.
Figures
Figure 1
Liquid-liquid phase separation contributes to RNP granule dynamics and eventual fibrillization. Within the cytosol, RNA binding proteins (RBPs) containing low complexity prion-like domains (LCDs) exist in an unaggregated state. Upon activating signaling cascades, the LCDs mediate weak aggregation through liquid-liquid phase separation, creating a distinct compartment enriched in RBPs, RNA and other associated proteins. Importantly, these ribonucleoprotein granules maintain the ability to exchange material with the cytosol while in this state, which allows them to carry out key mRNA regulating events. However, persistent aggregation over time or other pathological insults can drive these RBPs to further aggregate into compact, stable fibrils. In this model, the fibrillar forms of these granules can no longer exchange material with the surrounding cytosol and effectively trap their components in the granule.
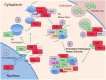
Figure 2
Interaction of Tau and RBP pathologies: (1) RBPs such as TIA1 splice RNA in the nucleus, and shuttle in and out of the nucleus. Although TIA1 is normally predominantly nuclear, Tau slows nuclear/cytoplasmic transport, decreasing anterograde transport less than retrograde transport, overall favoring a cytoplasmic localization of TIA1. (2) Tau binds to the small subunit of ribosomes, and this interaction changes in taupathies such as AD, resulting in stalled translation. Stalled translation initiation complexes containing mRNA and the small ribosomal subunit forms complexes with ribosomal proteins, mRNA, and core SG nucleating RBPs. (3) Small core stress granules forms complexes with additional RBPs including DDX helicases. (4) Tau increases the size of stress granules, and influences the specificity of RBPs included in stress granules. RBPs in complex with Tau and TIA1 include TAF15 and EWSR1. (5) TIA1 mediates interaction of stress granules with insoluble tau aggregates internalized from the cytoplasm (Meier et al., ; Brunello et al., ; Vanderweyde et al., 2016).
Similar articles
- Molecular interaction of stress granules with Tau and autophagy in Alzheimer's disease.
Yu QY, Ye LQ, Li HL. Yu QY, et al. Neurochem Int. 2022 Jul;157:105342. doi: 10.1016/j.neuint.2022.105342. Epub 2022 Apr 21. Neurochem Int. 2022. PMID: 35461975 Review. - Regulated protein aggregation: stress granules and neurodegeneration.
Wolozin B. Wolozin B. Mol Neurodegener. 2012 Nov 20;7:56. doi: 10.1186/1750-1326-7-56. Mol Neurodegener. 2012. PMID: 23164372 Free PMC article. Review. - Physiological protein aggregation run amuck: stress granules and the genesis of neurodegenerative disease.
Wolozin B. Wolozin B. Discov Med. 2014 Jan;17(91):47-52. Discov Med. 2014. PMID: 24411700 Free PMC article. Review. - Altered mRNP granule dynamics in FTLD pathogenesis.
Bowden HA, Dormann D. Bowden HA, et al. J Neurochem. 2016 Aug;138 Suppl 1:112-33. doi: 10.1111/jnc.13601. Epub 2016 Jun 15. J Neurochem. 2016. PMID: 26938019 Review. - Stress granules in neurodegeneration--lessons learnt from TAR DNA binding protein of 43 kDa and fused in sarcoma.
Bentmann E, Haass C, Dormann D. Bentmann E, et al. FEBS J. 2013 Sep;280(18):4348-70. doi: 10.1111/febs.12287. Epub 2013 May 9. FEBS J. 2013. PMID: 23587065 Review.
Cited by
- Axonal mRNA translation in neurological disorders.
Lin JQ, van Tartwijk FW, Holt CE. Lin JQ, et al. RNA Biol. 2021 Jul;18(7):936-961. doi: 10.1080/15476286.2020.1822638. Epub 2020 Sep 29. RNA Biol. 2021. PMID: 32988274 Free PMC article. Review. - The Biomarker and Therapeutic Potential of Circular Rnas in Schizophrenia.
Nedoluzhko A, Gruzdeva N, Sharko F, Rastorguev S, Zakharova N, Kostyuk G, Ushakov V. Nedoluzhko A, et al. Cells. 2020 Oct 4;9(10):2238. doi: 10.3390/cells9102238. Cells. 2020. PMID: 33020462 Free PMC article. Review. - Microarray analysis of verbenalin-treated human amniotic epithelial cells reveals therapeutic potential for Alzheimer's Disease.
Ferdousi F, Kondo S, Sasaki K, Uchida Y, Ohkohchi N, Zheng YW, Isoda H. Ferdousi F, et al. Aging (Albany NY). 2020 Mar 29;12(6):5516-5538. doi: 10.18632/aging.102985. Epub 2020 Mar 29. Aging (Albany NY). 2020. PMID: 32224504 Free PMC article. - RNA-binding proteins with basic-acidic dipeptide (BAD) domains self-assemble and aggregate in Alzheimer's disease.
Bishof I, Dammer EB, Duong DM, Kundinger SR, Gearing M, Lah JJ, Levey AI, Seyfried NT. Bishof I, et al. J Biol Chem. 2018 Jul 13;293(28):11047-11066. doi: 10.1074/jbc.RA118.001747. Epub 2018 May 25. J Biol Chem. 2018. PMID: 29802200 Free PMC article. - Cirbp-PSD95 axis protects against hypobaric hypoxia-induced aberrant morphology of hippocampal dendritic spines and cognitive deficits.
Zhou Y, Lu H, Liu Y, Zhao Z, Zhang Q, Xue C, Zou Y, Cao Z, Luo W. Zhou Y, et al. Mol Brain. 2021 Aug 21;14(1):129. doi: 10.1186/s13041-021-00827-1. Mol Brain. 2021. PMID: 34419133 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous