Recruitment of endosomal signaling mediates the forskolin modulation of guinea pig cardiac neuron excitability - PubMed (original) (raw)
Recruitment of endosomal signaling mediates the forskolin modulation of guinea pig cardiac neuron excitability
Jean C Hardwick et al. Am J Physiol Cell Physiol. 2017.
Abstract
Forskolin, a selective activator of adenylyl cyclase (AC), commonly is used to establish actions of G protein-coupled receptors (GPCRs) that are initiated primarily through activation of AC/cAMP signaling pathways. In the present study, forskolin was used to evaluate the potential role of AC/cAMP, which is a major signaling mechanism for the pituitary adenylate cyclase-activating polypeptide (PACAP)-selective PAC1 receptor, in the regulation of guinea pig cardiac neuronal excitability. Forskolin (5-10 µM) increases excitability in ~60% of the cardiac neurons. The forskolin-mediated increase in excitability was considered related to cAMP regulation of a cyclic nucleotide gated channel or via protein kinase A (PKA)/ERK signaling, mechanisms that have been linked to PAC1 receptor activation. However, unlike PACAP mechanisms, forskolin enhancement of excitability was not significantly reduced by treatment with cesium to block currents through hyperpolarization-activated nonselective cation channels (_I_h) or by treatment with PD98059 to block MEK/ERK signaling. In contrast, treatment with the clathrin inhibitor Pitstop2 or the dynamin inhibitor dynasore eliminated the forskolin-induced increase in excitability; treatments with the inactive Pitstop analog or PP2 treatment to inhibit Src-mediated endocytosis mechanisms were ineffective. The PKA inhibitor KT5702 significantly suppressed the forskolin-induced change in excitability; further, KT5702 and Pitstop2 reduced the forskolin-stimulated MEK/ERK activation in cardiac neurons. Collectively, the present results suggest that forskolin activation of AC/cAMP/PKA signaling leads to the recruitment of clathrin/dynamin-dependent endosomal transduction cascades, including MEK/ERK signaling, and that endosomal signaling is the critical mechanism underlying the forskolin-induced increase in cardiac neuron excitability.
Keywords: MAPK signaling; PKA; autonomic neuron; forskolin; neuronal excitability.
Copyright © 2017 the American Physiological Society.
Figures
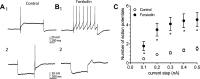
Fig. 1.
Forskolin can increase cardiac neuron excitability and the rectification in hyperpolarizations elicited by constant current injection. A1 and B1: example recording of action potentials elicited by a 1,000 ms, 0.3 nA depolarizing step before (A1) and during 5 µM forskolin exposure (B1). A2 and B2: the slight sag (rectification) in the hyperpolarization induced by the constant current step to ~−90 mV (A2) is due to the activation of the hyperpolarization-activated inward current, _I_h. This rectification is more evident during 5 µM forskolin exposure (B2), indicative of an enhanced _I_h. In this cell, a rebound depolarization followed the termination of the hyperpolarization, which in A2, but not B2, was sufficient in magnitude to elicit an action potential. The resting membrane potential was initially −60 mV, but over the course of the recording, the cell hyperpolarized by a few millivolts such that the rebound depolarization, although still evident, did not reach the threshold for action potential generation. C: averaged excitability curves for different cells before and during exposure to 5 µM forskolin. ○, Averaged excitability curve for 18 cells before forskolin; ●, averaged excitability curve for these same cells during exposure to 5 µM forskolin; *, number of action potentials elicited were significantly greater in forskolin-treated than in control cells.
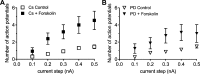
Fig. 2.
Neither cesium nor PD98059 pretreatment significantly decreases the forskolin-induced increase in excitability. A: averaged excitability curves for cells pretreated with 1 mM cesium (Cs) and then exposed to Cs and 5 µM forskolin. □, Averaged excitability curve for 15 cells during treatment with cesium alone; ■, averaged excitability curve for 15 cells during exposure to cesium and forskolin. B: averaged excitability curves for cells pretreated with 50 µM PD98059 (PD) and then exposed to PD98059 and 5 µM forskolin. ∇, Averaged excitability curve for 8 cells during pretreatment with PD98059 alone; ▼, averaged excitability curve for the same 8 cells during exposure to PD98059 and forskolin. From comparison of the excitability curves in A (Cs and forskolin) and B (PD and forskolin), with the excitability curve for forskolin alone in Fig. 1_C_, it was determined that neither cesium nor PD98059 significantly decreased the number of action potentials elicited by each depolarizing step. Furthermore, the averaged excitability curves determined before forskolin exposure were the same for control cells or cells just exposed to cesium or PD98059.
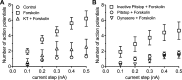
Fig. 3.
Pretreatment with KT5702, an inhibitor of PKA, and inhibitors of clathrin/dynamin-mediated endocytosis blunts the forskolin-induced increase in excitability. A: 1 µM KT5702 pretreatment significantly depresses the forskolin-induced increase in excitability. ○, Averaged excitability curve for 17 control cells; □, averaged excitability curve for 18 cells exposed to 10 µM forskolin. △, averaged excitability curve for 9 cells pretreated with 1 µM KT5702 and then exposed to KT5702 and 10 µM forskolin. The number of action potentials generated by each depolarizing step was significantly less (P < 0.05, unpaired _t_-test) in cells exposed to KT5702 and forskolin than for cells exposed to forskolin alone. B: pretreatment with Pitstop2 and dynasore, but not the inactive analog of Pitstop, reduces the 10 µM forskolin-induced increase in excitability. □, Averaged excitability curve for 12 cells pretreated with the inactive analog of Pitstop (15 µM) and then exposed to the Pitstop inactive analog and 10 µM forskolin; ○, averaged excitability curve for 16 cells pretreated with Pitstop2 and then exposed to Pitstop2 and 10 µM forskolin; ∇, averaged excitability curve for 10 cells pretreated with 20 µM dynasore and then exposed to dynasore and 10 µM forskolin. The number of action potentials generated by each depolarizing step was significantly less (P < 0.05, one-way ANOVA) in cells exposed to Pitstop2 and forskolin or dynasore and forskolin than for cells exposed to the inactive analog of Pitstop.
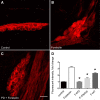
Fig. 4.
Pitstop2 and KT5702 decrease the forskolin-induced generation of neuronal pERK in cardiac neurons. A_–_C: examples of pERK-immunoreactivity in cardiac neurons under control conditions (A), following a 20-min exposure to 10 µM forskolin (B), and following a 20-min exposure to 10 µM forskolin and 50 µM PD98059 that was preceded by a 15-min pretreatment with 50 µM PD98059 (C). Calibration in C equals 20 µm. D: the forskolin-induced increase in pERK generation is eliminated by pretreatment with 50 µM PD98059 (F+PD) and reduced by pretreatment with 15 µM Pitstop2 (F+Pitstop) or 1 µM KT5702 (F+KT). Results are summarized from multiple cells in different cardiac ganglia whole mount preparations: Control, 53 cells in 4 whole mounts; forskolin, 88 cells in 3 whole mounts; PD98059/forskolin, 30 cells in 2 whole mounts; Pitstop2/forskolin, 58 cells in 3 whole mounts, and KT5702/forskolin, 47 cells in 2 whole mounts. Treatment with PD98059, Pitstop2, or KT5702 all significantly reduced the forskolin-induced generation of pERK (*P < 0.05 by one-way ANOVA).
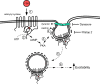
Fig. 5.
Forskolin (FSK) recruits endosomal signaling to enhance neuronal excitability. This schematic summarizes the results obtained in the present study, which support the hypothesis that forskolin recruits endosomal signaling. Forskolin stimulation of adenylyl cyclase (1) results in the catalysis of ATP to cAMP (2), leading to the activation of PKA (3). PKA facilitates clathrin and dynamin accumulation (4) to initiate plasma membrane endocytosis, creating a signaling endosome (5). The endosome becomes a platform for the recruitment of downstream signaling cascades, including MEK/ERK and other unidentified transduction cascades (6) that modulate ionic conductances regulating neuronal excitability.
Similar articles
- Src family kinase inhibitors blunt PACAP-induced PAC1 receptor endocytosis, phosphorylation of ERK, and the increase in cardiac neuron excitability.
Tompkins JD, Clason TA, Buttolph TR, Girard BM, Linden AK, Hardwick JC, Merriam LA, May V, Parsons RL. Tompkins JD, et al. Am J Physiol Cell Physiol. 2018 Feb 1;314(2):C233-C241. doi: 10.1152/ajpcell.00223.2017. Epub 2017 Nov 15. Am J Physiol Cell Physiol. 2018. PMID: 29141923 Free PMC article. - Activation of MEK/ERK Signaling by PACAP in Guinea Pig Cardiac Neurons.
Clason TA, Girard BM, May V, Parsons RL. Clason TA, et al. J Mol Neurosci. 2016 Jun;59(2):309-16. doi: 10.1007/s12031-016-0766-z. Epub 2016 May 18. J Mol Neurosci. 2016. PMID: 27194157 Free PMC article. - PACAP-Induced PAC1 Receptor Internalization and Recruitment of Endosomal Signaling Regulate Cardiac Neuron Excitability.
Parsons RL, May V. Parsons RL, et al. J Mol Neurosci. 2019 Jul;68(3):340-347. doi: 10.1007/s12031-018-1127-x. Epub 2018 Jul 27. J Mol Neurosci. 2019. PMID: 30054797 Free PMC article. Review. - Activation of MEK/ERK signaling contributes to the PACAP-induced increase in guinea pig cardiac neuron excitability.
Tompkins JD, Clason TA, Hardwick JC, Girard BM, Merriam LA, May V, Parsons RL. Tompkins JD, et al. Am J Physiol Cell Physiol. 2016 Oct 1;311(4):C643-C651. doi: 10.1152/ajpcell.00164.2016. Epub 2016 Aug 3. Am J Physiol Cell Physiol. 2016. PMID: 27488668 Free PMC article. - PAC1 Receptor Internalization and Endosomal MEK/ERK Activation Is Essential for PACAP-Mediated Neuronal Excitability.
May V, Johnson GC, Hammack SE, Braas KM, Parsons RL. May V, et al. J Mol Neurosci. 2021 Aug;71(8):1536-1542. doi: 10.1007/s12031-021-01821-x. Epub 2021 Mar 6. J Mol Neurosci. 2021. PMID: 33675454 Free PMC article. Review.
Cited by
- Pituitary adenylate cyclase-activating polypeptide receptor activation in the hypothalamus recruits unique signaling pathways involved in energy homeostasis.
Maunze B, Bruckner KW, Desai NN, Chen C, Chen F, Baker D, Choi S. Maunze B, et al. Am J Physiol Endocrinol Metab. 2022 Mar 1;322(3):E199-E210. doi: 10.1152/ajpendo.00320.2021. Epub 2022 Jan 10. Am J Physiol Endocrinol Metab. 2022. PMID: 35001657 Free PMC article. - Src family kinase inhibitors blunt PACAP-induced PAC1 receptor endocytosis, phosphorylation of ERK, and the increase in cardiac neuron excitability.
Tompkins JD, Clason TA, Buttolph TR, Girard BM, Linden AK, Hardwick JC, Merriam LA, May V, Parsons RL. Tompkins JD, et al. Am J Physiol Cell Physiol. 2018 Feb 1;314(2):C233-C241. doi: 10.1152/ajpcell.00223.2017. Epub 2017 Nov 15. Am J Physiol Cell Physiol. 2018. PMID: 29141923 Free PMC article.
References
- Brodsky FM, Sosa RT, Ybe JA, O’Halloran TJ. Unconventional functions for clathrin ESCRTs, and other endocytic regulators in the cytoskeleton, cell cycle, nucleus, and beyond: links to human disease. Cold Spring Harb Perspect Biol 2014: a0117004, 2016. doi:10.1101/cshperspect.a017004. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous