An immunogenic personal neoantigen vaccine for patients with melanoma - PubMed (original) (raw)
Clinical Trial
. 2017 Jul 13;547(7662):217-221.
doi: 10.1038/nature22991. Epub 2017 Jul 5.
Patrick A Ott 1 2 3, Derin B Keskin 1 3 4, Sachet A Shukla 1 4, Jing Sun 1, David J Bozym 1, Wandi Zhang 1, Adrienne Luoma 5, Anita Giobbie-Hurder 6, Lauren Peter 7 8, Christina Chen 1, Oriol Olive 1, Todd A Carter 4, Shuqiang Li 4, David J Lieb 4, Thomas Eisenhaure 4, Evisa Gjini 9, Jonathan Stevens 10, William J Lane 10, Indu Javeri 11, Kaliappanadar Nellaiappan 11, Andres M Salazar 12, Heather Daley 1, Michael Seaman 7, Elizabeth I Buchbinder 1 2 3, Charles H Yoon 3 13, Maegan Harden 4, Niall Lennon 4, Stacey Gabriel 4, Scott J Rodig 9 10, Dan H Barouch 3 7 8, Jon C Aster 3 10, Gad Getz 3 4 1, Kai Wucherpfennig 3 5, Donna Neuberg 6, Jerome Ritz 1 2 3, Eric S Lander 3 4, Edward F Fritsch 1 4, Nir Hacohen 3 4 1, Catherine J Wu 1 2 3 4
Affiliations
- PMID: 28678778
- PMCID: PMC5577644
- DOI: 10.1038/nature22991
Clinical Trial
An immunogenic personal neoantigen vaccine for patients with melanoma
Patrick A Ott et al. Nature. 2017.
Erratum in
- Corrigendum: An immunogenic personal neoantigen vaccine for patients with melanoma.
Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, Chen C, Olive O, Carter TA, Li S, Lieb DJ, Eisenhaure T, Gjini E, Stevens J, Lane WJ, Javeri I, Nellaiappan K, Salazar AM, Daley H, Seaman M, Buchbinder EI, Yoon CH, Harden M, Lennon N, Gabriel S, Rodig SJ, Barouch DH, Aster JC, Getz G, Wucherpfennig K, Neuberg D, Ritz J, Lander ES, Fritsch EF, Hacohen N, Wu CJ. Ott PA, et al. Nature. 2018 Mar 14;555(7696):402. doi: 10.1038/nature25145. Nature. 2018. PMID: 29542692 Free PMC article.
Abstract
Effective anti-tumour immunity in humans has been associated with the presence of T cells directed at cancer neoantigens, a class of HLA-bound peptides that arise from tumour-specific mutations. They are highly immunogenic because they are not present in normal tissues and hence bypass central thymic tolerance. Although neoantigens were long-envisioned as optimal targets for an anti-tumour immune response, their systematic discovery and evaluation only became feasible with the recent availability of massively parallel sequencing for detection of all coding mutations within tumours, and of machine learning approaches to reliably predict those mutated peptides with high-affinity binding of autologous human leukocyte antigen (HLA) molecules. We hypothesized that vaccination with neoantigens can both expand pre-existing neoantigen-specific T-cell populations and induce a broader repertoire of new T-cell specificities in cancer patients, tipping the intra-tumoural balance in favour of enhanced tumour control. Here we demonstrate the feasibility, safety, and immunogenicity of a vaccine that targets up to 20 predicted personal tumour neoantigens. Vaccine-induced polyfunctional CD4+ and CD8+ T cells targeted 58 (60%) and 15 (16%) of the 97 unique neoantigens used across patients, respectively. These T cells discriminated mutated from wild-type antigens, and in some cases directly recognized autologous tumour. Of six vaccinated patients, four had no recurrence at 25 months after vaccination, while two with recurrent disease were subsequently treated with anti-PD-1 (anti-programmed cell death-1) therapy and experienced complete tumour regression, with expansion of the repertoire of neoantigen-specific T cells. These data provide a strong rationale for further development of this approach, alone and in combination with checkpoint blockade or other immunotherapies.
Conflict of interest statement
Competing Financial Interests (Online only)
E.F.F is a founder and employee of Neon Therapeutics; N.H. and C.J.W. are founders of Neon Therapeutics and members of its scientific advisory board. P.A.O. has advised Neon Therapeutics. E.S.L. is a founder of Neon Therapeutics and a member of the Board of Directors. K.N. and I.J. are employees of CuriRx. Patent applications have been filed on aspects of the described work entitled as follows: Compositions and Methods for Personalized Neoplasia Vaccines (N.H., E.F.F., and C.J.W.), Methods for Identifying Tumor Specific Neo-Antigens (N.H. and C.J.W.), Formulations for Neoplasia Vaccines (E.F.F., K.N., and I.J.) and Combination Therapy for Neoantigen Vaccine (N.H., C.J.W., and E.F.F). S.J.R. receives research funding from Bristol-Myers Squibb, MedImmune and is on the scientific advisory board for Perkin Elmer Inc. The remaining authors declare no competing financial interests.
Figures
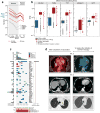
Extended Data Figure 1. Mutational landscape of patient melanoma and radiographic evidence of complete response following anti-PD-1 therapy for Patients 2 and 6
a, Numbers of mutations and predicted epitopes/ patient tumor. Red solid lines—patients with vaccines generated and vaccinations completed (n=6); red dotted—vaccines generated but vaccinations not initiated (n=2); grey— insufficient mutation number for vaccine generation (n=2) b, Expression (measured and normalized as transcripts per million base pairs [TPM] from the RNA-Seq data) of known melanoma-associated genes in the tumor specimens for all 10 patients entered to the trial (circles) compared to normal tissue (blue box, [GTEx data]) and melanoma from TCGA (red box; see [Methods for analysis]). c, The overall mutational landscape of the 8 patient melanoma samples for which immunizing peptides were generated (# mutations/Mb [top]; distribution of nucleotide changes [bottom]), and the presence of mutations in genes previously identified as significant in melanoma TCGA samples (n=290) by the MutSig2CV algorithm ([middle] doi:10.7908/C1J67GCG; genes ordered based on significance of recurrence reported per TCGA, Supplementary Information 2). d, Patient 2, left panel: Positron emission tomography computed tomography (PET/CT) scans obtained 8 weeks after the last booster vaccine demonstrate new intensely fludeoxyglucose F 18 (FDG)-avid right hilar lymphadenopathy measuring ~2.1cm (Maximal Standardized Uptake Value: 20.3 [yellow arrow]). Right panel: PET/CT obtained 12 weeks later, after 4 doses of pembrolizumab at 2mg/kg given every 3 weeks, revealing complete interval resolution of the right hilar lymphadenopathy consistent with a complete response. Patient 6, left panels: CT scans of the chest obtained 1 week after the last booster vaccine demonstrate multiple soft tissue nodules along the left lateral and posterolateral chest wall (left upper panel, yellow arrow indicates a 2.1×1.8 soft tissue nodule as an example) and a left lower lobe pulmonary nodule (left lower panel, yellow arrow). Right panels: CT scans obtained 16.5 weeks later, after 4 doses of pembrolizumab at 2mg/kg given every 3 weeks, revealing complete interval resolution of all lesions consistent with a complete response. On the upper panels, note that the liver is partially visualized on the pre-treatment scan (left upper panel) as a result of expirational state and not visualized on the post-treatment scan (right upper panel) as a result of inspirational state. The cross sections visualizing the soft tissue metastases in the left chest wall versus their resolution after treatment correspond. Pt: patient.
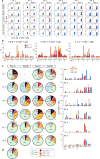
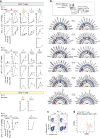
Extended Data Figure 2. Polyfunctional neoantigen-specific CD4+ T cell responses are induced by vaccination in all 6 patients
Frequencies of CD4+ T cells secreting cytokines in response to pools of 15–16mer assay peptides (ASP), as measured by intracellular cytokine staining (ICS) after stimulation of PBMCs ex vivo with ASP pools. a, IFN-γ-producing CD4+ T cells detected by flow cytometry pre-vaccination and at week 16 post-vaccination for all 6 vaccinated patients. FACS plots were pre-gated on CD4+ T cells. Red - frequencies of ASP pool reactive IFN-γ+ cells as a proportion of all CD4+ T cells. b, Frequencies of IFN-γ, TNF-α and IL-2 producing cells within CD4+ T cells in response to ASP pools tested in samples collected pre-vaccination (blue) and at week 16 post-vaccination (red). c, Pie charts of the total CD4+ T cell responses positive for one, two or three cytokines. Bar graphs are shown of absolute frequencies of ASP pool reactive CD4+ T cells producing one, two, or three cytokines. d, Median percent cytokine production across 6 patients by CD4+ T cells ex vivo against ASP peptide pools.
Extended Data Figure 3. Polyfunctional neoantigen-specific CD8+ T cell responses are induced by vaccination in all 6 patients
Frequencies of CD8+ T cells secreting cytokines in response to pools of 9–10mer predicted class I epitope peptides (EPT) after a single round of pre-stimulation. PBMCs were cultured with EPT pools for 10–21 days followed by ICS. a, IFN-γ-producing CD8+ T cells detected by flow cytometry pre-vaccination and at week 16 post-vaccination for all 6 vaccinated patients. FACS plots were pre-gated on CD8+ T cells. Percentages shown in red are frequencies of EPT pool reactive IFN-γ+ cells as a proportion of all CD8+ T cells. b, IFN-γ responses of CD8+ T cells against EPT pools after one round of pre-stimulation measured by ICS pre-vaccination and at 16 weeks post-vaccination. c, Bar graphs show absolute frequencies of EPT pool reactive CD8+ T cells producing one, two, or three cytokines. Pie charts summarize the fractions of the total CD8+ T cell response that are positive for one, two or three cytokines. d, Median percent cytokine production across 6 patients by CD8+ T cells following one round of pre-stimulation against EPT peptide pools.
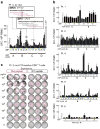
Extended Data Figure 4. Deconvolution of peptide pools to identify neoantigen-specific T cell responses ex vivo against individual 15–16mer ASP
Deconvolution of T cell reactivity against individual ASP by ex vivo IFN-γ ELISPOT using the week 16 post-vaccination PBMCs, tested in duplicate wells per peptide (bars: SEM). Responses were scored positive (‘*’) if >55 SFC (spot forming cell) were detected and were at least 1.5 standard deviations (SD) over the DMSO control (> 3 SD over background for Pt. 5 and 6). a, Patient 3 deconvolution. Inset: examples of positive IMP/ASP sequences; red- ASP stimulating reactivity, boxed- the mutated aa. b, Deconvolution for all other patients. c, IFN-γ ELISPOT response of Patient 3 _CIT_-specific CD8+ T cell line from week 16 (7×104 CD8+ T cells/well) against mutated (Mut) versus corresponding wildtype (WT) peptide across a range of concentrations (10pg/ml–10µg/ml) pulsed on 1×104 T cell-depleted PBMCs (hereafter referred to as APCs)/well, tested in triplicate.
Extended Data Figure 5. Mapping of CD4+ and CD8+ T cell responses to individual ASP and EPT to the immunizing peptides (IMP) for all 6 patients
ASP and EPT covering the immunizing peptides are shown for the IMP that induced T cell responses. T cells from week 16 PBMCs were tested. Red bold and shading – to indicate mutated amino acid; absent in those IMPs arising from neoORFs. Blue underline – for class I epitopes, predicted epitopes (IC50< 300nM) based on NetMHCpan,; for class II epitopes, predicted epitopes <10 percentile based on the IEDB-recommended consensus approach combining NN-align, SMM-align, and CombLib if allele predictions are available, otherwise NetMHCIIpan (Supplementary Information 6). Red font– peptides that generated an ex vivo CD4+ T cell responses; blue font– peptides that generated T cell response after 1 round of pre-stimulation with peptides, Triangle– ASP or EPT-specific T cells that also recognized a corresponding mutated minigene; *– T cell responses against minigenes that were blocked by pan anti- HLA-DR or anti-HLA class I blocking antibodies for CD4+ or CD8+ T cells, respectively.
Extended Data Figure 6. IFN-γ response of pre- and post-vaccination PBMC against individual ASP; IFN-γ response and CD107αβ degranulation by neoantigen-specific T cell response against expressed minigenes
PBMCs from week 16 post-vaccination were pre-stimulated with ASP and EPT for measuring CD4+ and CD8+ T cell responses, respectively. a, Detection of IFN-γ responses by ELISPOT of CD4+ T cells against individual ASP after one round of pre-stimulation with ASP pools in matched pre- and 16 weeks post-vaccination samples for Patients 1–6 (1×104 APCs and 1×104 T cells/well), tested in duplicate or triplicate wells per peptide (bars: SEM). b, Autologous B cells were nucleofected with in vitro translated RNA generated from Mut or WT minigenes (‘MG’). T cell lines were cultured with Mut or WT minigene nucleofected B cells, Mut minigene nucleofected B cells with anti-HLA class I or anti-HLA DR antibodies, non-nucleofected B cells with DMSO in IFN-γ ELISPOT (8×104 B cells/well were plated with 5×103 T cells/well and 1×104 CD8+ T cells/well for CD4+ and CD8+ T cell response detection, respectively), tested in duplicate or triplicate wells per each condition (bars: SEM). c, T cell lines were cultured with Mut minigene nucleofected B cells or non-nucleofected B cells followed by CD107αβ degranulation assay. Representative FACS plot of Patient 3 CD107αβ degranulation assay for _VPS16_-specific CD8+ T cell lines (top) and other neoantigen-specific CD4+ and CD8+ T cell lines (bottom) from week 16 against autologous B cells nucleofected or not with mutated minigenes. FACS plot were pre-gated on CD8+ T cells.
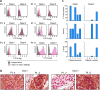
Extended Data Figure 7. Surface expression of HLA class I and class II on patient melanoma cell lines and originally resected tumor
a, Flow cytometric staining of autologous melanoma cell lines generated from primary tumor samples with anti-class I and class II or isotype antibodies. b, Dual chromogenic immunohistochemical (IHC) staining of excised FFPE tumors (see Methods for details). Representative images of positive staining for HLA class I (Pt. 4 and 2) and HLA class II (Pt. 6) and negative staining for class II (Pt. 2). Red- melanoma transcription factor SOX10, brown- HLA class I or class II. c, Summary of IHC results of 5 patients with available FFPE tissue. Semi-quantitative scoring was performed for the intensity of positive staining of melanoma cell membranes for class I or II (0, negative; 1, weak; 2, moderate; 3, strong) and for the percentage of positive staining malignant cells (0–100%). A cumulative H score was obtained by multiplying intensity score by the percentage of malignant cells with positive staining.
Extended Data Figure 8. Repertoire of neoantigen-specific T cell responses persists and broadens following PD-1 blockade; summary of all CD4+ and CD8+ T cell responses against neoantigens across the 6 patients
a, Persistent or new responses to mutated peptides following PD-1 blockade (Pembrolizumab) are shown. PBMCs from Patients 2 and 6 (pre-vaccination, week 16 post-vaccination or 11.5 months [Patient 2] or 8.5 months [Patient 6] after initiation of pembrolizumab therapy) were pre-stimulated for 21 days with ASP or EPT pools, and reactivity against individual ASP and EPT were then tested by IFN-γ ELISPOT. 1×104 APCs/well were plated with 1×104 T cell lines/well and 1×104 CD8+ T cell lines/well for CD4+ and CD8+ T cell response detection, respectively, tested in duplicate or triplicate wells per peptide (bars indicate SEM). Each line represents IFN-γ response against a single ASP or EPT tested per immunizing peptide. (#) for each gene – total number of ASP or EPT tested per immunizing peptide. b, The rings, from outer to inner, show: (1) predicted affinity of peptide for HLA. Heat map scaled from darkest (lowest IC50 [nM], or highest affinity) to lightest (highest IC50 [nM], or lowest affinity); (2) level of transcript expression of the tumor, scaled from darkest (highest number of transcripts per million reads [TPM] ) mapped to lightest (lowest TPM); (3) reactivity to peptide-pulsed autologous APCs; (4) reactivity to B cells nucleofected with minigenes; (5) reactivity to cultured autologous melanoma cells. c, d, Ex vivo class II tetramer staining of Patient 1 CD4+ T cells. Visualization of transcriptomes of single CD4+ T cells pre-vaccination and tetramer-positive CD4+ cells post-vaccination by tSNE.
Figure 1. Generation of a personal, multi-peptide neoantigen vaccine for patients with high-risk melanoma
a, Somatic mutations were identified by WES of melanoma and germline DNA and their expression confirmed by tumor RNA-sequencing. Immunizing peptides were selected based on HLA binding predictions (Methods). Each patient received up to 20 long peptides in 4 pools. b, Clinical event timeline for 6 vaccinated patients from surgery until time of data cutoff (36 months from study initiation).
Figure 2. Vaccination induces strong multi-functional CD4+ and CD8+ T cell responses in high-risk melanoma patients
a, Schema of immunizing (IMP), assay (ASP) and epitope (EPT) peptides. Mutated amino acid (aa) shaded. b, Ex vivo IFN-γ ELISPOT of PBMC, with duplicate or triplicate wells/timepoint (bars: SEM; Methods for statistical analysis) c, Ex vivo intracellular cytokine staining (ICS) of Patient 3 PBMC pre- and post-vaccination, pre-gated on CD3+ T cells. d, Patient 1 CD8+ T cell responses after one round of pre-stimulation with EPT peptide pools.
Figure 3. Vaccine-induced T cells discriminate mutated from wildtype antigens and detect endogenously processed and presented peptides
a, IFN-γ secretion by neoantigen-reactive T cell lines against mutated and wildtype peptides, focusing on neoantigens generating ex vivo CD4+ and pre-stimulated CD8+ T cell responses (bars: SEM). b, ICS of CD8+ T cell lines stimulated with EPT peptides pre- and post-vaccination. c, IFN-γ secretion by Patient 3 neoantigen-specific T cell lines against minigene (MG)-nucleofected B cells, with/without anti-HLA class I or DR antibodies. d, e, IFN-γ secretion by Patient 6 and Patient 2 neoantigen-specific T cell lines against autologous tumor. f, IFN-γ secretion by Patient 5 neoantigen-specific CD4+ T cells against autologous dendritic cells co-cultured with irradiated autologous melanoma. All T cell lines originated from week 16 PBMC; ELISPOT experiments were performed in duplicate or triplicate wells/condition.
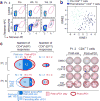
Figure 4. Vaccine-induced T cells demonstrate broad shifts in their transcriptional programs; the repertoire of neoantigen-specific T cells persists and broadens following PD-1 blockade
a, b, Ex vivo class II tetramer staining of Patient 4 CD4+ T cells. Visualization of transcriptomes of single CD4+ T cells pre-vaccination and tetramer-positive CD4+ cells post-vaccination by tSNE. c, Summary of neoantigen-specific T cell responses after vaccination and after PD-1 blockade for Patients 2 and 6. Example IFN-γ ELISPOT results are shown for week 16 post-vaccination and post-PD-1 CD4+ T cells against mut-CDK13, -JPH1, and –ADM2d for Patient 2. For complete response kinetics, see Extended Data Fig. 8a.
Comment in
- Cancer: Precision T-cell therapy targets tumours.
Melief CJM. Melief CJM. Nature. 2017 Jul 13;547(7662):165-167. doi: 10.1038/nature23093. Epub 2017 Jul 5. Nature. 2017. PMID: 28678783 No abstract available. - Tumour vaccines: Personal training by vaccination.
Harjes U. Harjes U. Nat Rev Cancer. 2017 Jul 25;17(8):451. doi: 10.1038/nrc.2017.61. Nat Rev Cancer. 2017. PMID: 28740114 No abstract available. - Making It Personal: Neoantigen Vaccines in Metastatic Melanoma.
Hellmann MD, Snyder A. Hellmann MD, et al. Immunity. 2017 Aug 15;47(2):221-223. doi: 10.1016/j.immuni.2017.08.001. Immunity. 2017. PMID: 28813655 - Neoantigen Vaccines Pass the Immunogenicity Test.
Linette GP, Carreno BM. Linette GP, et al. Trends Mol Med. 2017 Oct;23(10):869-871. doi: 10.1016/j.molmed.2017.08.007. Epub 2017 Aug 31. Trends Mol Med. 2017. PMID: 28867556 Free PMC article.
Similar articles
- Immunological ignorance is an enabling feature of the oligo-clonal T cell response to melanoma neoantigens.
Linette GP, Becker-Hapak M, Skidmore ZL, Baroja ML, Xu C, Hundal J, Spencer DH, Fu W, Cummins C, Robnett M, Kaabinejadian S, Hildebrand WH, Magrini V, Demeter R, Krupnick AS, Griffith OL, Griffith M, Mardis ER, Carreno BM. Linette GP, et al. Proc Natl Acad Sci U S A. 2019 Nov 19;116(47):23662-23670. doi: 10.1073/pnas.1906026116. Epub 2019 Nov 4. Proc Natl Acad Sci U S A. 2019. PMID: 31685621 Free PMC article. - A Phase Ib Trial of Personalized Neoantigen Therapy Plus Anti-PD-1 in Patients with Advanced Melanoma, Non-small Cell Lung Cancer, or Bladder Cancer.
Ott PA, Hu-Lieskovan S, Chmielowski B, Govindan R, Naing A, Bhardwaj N, Margolin K, Awad MM, Hellmann MD, Lin JJ, Friedlander T, Bushway ME, Balogh KN, Sciuto TE, Kohler V, Turnbull SJ, Besada R, Curran RR, Trapp B, Scherer J, Poran A, Harjanto D, Barthelme D, Ting YS, Dong JZ, Ware Y, Huang Y, Huang Z, Wanamaker A, Cleary LD, Moles MA, Manson K, Greshock J, Khondker ZS, Fritsch E, Rooney MS, DeMario M, Gaynor RB, Srinivasan L. Ott PA, et al. Cell. 2020 Oct 15;183(2):347-362.e24. doi: 10.1016/j.cell.2020.08.053. Cell. 2020. PMID: 33064988 Clinical Trial. - HLA class I loss in metachronous metastases prevents continuous T cell recognition of mutated neoantigens in a human melanoma model.
Schrörs B, Lübcke S, Lennerz V, Fatho M, Bicker A, Wölfel C, Derigs P, Hankeln T, Schadendorf D, Paschen A, Wölfel T. Schrörs B, et al. Oncotarget. 2017 Apr 25;8(17):28312-28327. doi: 10.18632/oncotarget.16048. Oncotarget. 2017. PMID: 28423700 Free PMC article. - Personalized neoantigen vaccines: A new approach to cancer immunotherapy.
Aldous AR, Dong JZ. Aldous AR, et al. Bioorg Med Chem. 2018 Jun 1;26(10):2842-2849. doi: 10.1016/j.bmc.2017.10.021. Epub 2017 Oct 19. Bioorg Med Chem. 2018. PMID: 29111369 Review. - Neoantigen vaccine: an emerging tumor immunotherapy.
Peng M, Mo Y, Wang Y, Wu P, Zhang Y, Xiong F, Guo C, Wu X, Li Y, Li X, Li G, Xiong W, Zeng Z. Peng M, et al. Mol Cancer. 2019 Aug 23;18(1):128. doi: 10.1186/s12943-019-1055-6. Mol Cancer. 2019. PMID: 31443694 Free PMC article. Review.
Cited by
- The state-of-the-art of N-of-1 therapies and the IRDiRC N-of-1 development roadmap.
Jonker AH, Tataru EA, Graessner H, Dimmock D, Jaffe A, Baynam G, Davies J, Mitkus S, Iliach O, Horgan R, Augustine EF, Bateman-House A, Pasmooij AMG, Yu T, Synofzik M, Douville J, Lapteva L, Brooks PJ, O'Connor D, Aartsma-Rus A; N-of-1 Task Force of the International Rare Diseases Research Consortium (IRDiRC). Jonker AH, et al. Nat Rev Drug Discov. 2024 Nov 4. doi: 10.1038/s41573-024-01059-3. Online ahead of print. Nat Rev Drug Discov. 2024. PMID: 39496921 Review. - Personalized mRNA Vaccines and Contemporary Melanoma Practice.
Hieken TJ, Ariyan C. Hieken TJ, et al. Ann Surg Oncol. 2024 Oct 31. doi: 10.1245/s10434-024-15731-w. Online ahead of print. Ann Surg Oncol. 2024. PMID: 39482478 No abstract available. - AML-VAC-XS15-01: protocol of a first-in-human clinical trial to evaluate the safety, tolerability and preliminary efficacy of a multi-peptide vaccine based on leukemia stem cell antigens in acute myeloid leukemia patients.
Jung S, Nelde A, Maringer Y, Denk M, Zieschang L, Kammer C, Özbek M, Martus P, Hackenbruch C, Englisch A, Heitmann JS, Salih HR, Walz JS. Jung S, et al. Front Oncol. 2024 Oct 14;14:1458449. doi: 10.3389/fonc.2024.1458449. eCollection 2024. Front Oncol. 2024. PMID: 39469638 Free PMC article. - Vax-Innate: improving therapeutic cancer vaccines by modulating T cells and the tumour microenvironment.
Baharom F, Hermans D, Delamarre L, Seder RA. Baharom F, et al. Nat Rev Immunol. 2024 Oct 21. doi: 10.1038/s41577-024-01091-9. Online ahead of print. Nat Rev Immunol. 2024. PMID: 39433884 Review. - CRISPR/Cas9 technology for advancements in cancer immunotherapy: from uncovering regulatory mechanisms to therapeutic applications.
Feng X, Li Z, Liu Y, Chen D, Zhou Z. Feng X, et al. Exp Hematol Oncol. 2024 Oct 19;13(1):102. doi: 10.1186/s40164-024-00570-y. Exp Hematol Oncol. 2024. PMID: 39427211 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- P50 CA101942/CA/NCI NIH HHS/United States
- R01 HL103532/HL/NHLBI NIH HHS/United States
- R50 CA211482/CA/NCI NIH HHS/United States
- R01 CA155010/CA/NCI NIH HHS/United States
- T32 CA207021/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials