The Dual Role of Microglia in ALS: Mechanisms and Therapeutic Approaches - PubMed (original) (raw)
Review
The Dual Role of Microglia in ALS: Mechanisms and Therapeutic Approaches
Maria Concetta Geloso et al. Front Aging Neurosci. 2017.
Abstract
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by a non-cell autonomous motor neuron loss. While it is generally believed that the disease onset takes place inside motor neurons, different cell types mediating neuroinflammatory processes are considered deeply involved in the progression of the disease. On these grounds, many treatments have been tested on ALS animals with the aim of inhibiting or reducing the pro-inflammatory action of microglia and astrocytes and counteract the progression of the disease. Unfortunately, these anti-inflammatory therapies have been only modestly successful. The non-univocal role played by microglia during stress and injuries might explain this failure. Indeed, it is now well recognized that, during ALS, microglia displays different phenotypes, from surveillant in early stages, to activated states, M1 and M2, characterized by the expression of respectively harmful and protective genes in later phases of the disease. Consistently, the inhibition of microglial function seems to be a valid strategy only if the different stages of microglia polarization are taken into account, interfering with the reactivity of microglia specifically targeting only the harmful pathways and/or potentiating the trophic ones. In this review article, we will analyze the features and timing of microglia activation in the light of M1/M2 phenotypes in the main mice models of ALS. Moreover, we will also revise the results obtained by different anti-inflammatory therapies aimed to unbalance the M1/M2 ratio, shifting it towards a protective outcome.
Keywords: M1/M2 microglia; amyotrophic lateral sclerosis; anti-inflammatory drugs; genetic modifiers; mutant SOD1 mice; neuroinflammation.
Figures
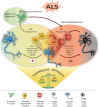
Figure 1
M1/M2 microglia polarization during amyotrophic lateral sclerosis (ALS)-induced motor neuron degeneration. During ALS progression activated microglia represent a continuum between the neuroprotective M2 phenotype, which promotes tissue repair and supports neuron survival by releasing neuroprotective factors, vs. the toxic M1, which produces cytokines increasing inflammation and further supporting M1 polarization, thus contributing to neuronal death. Therapeutic approaches targeting microglia polarization and resulting in induction of the M2 phenotype are promising strategies to ameliorate local neurodegeneration and improve the clinical outcome of the disease (see Table 1 for details).
Similar articles
- Clemastine Confers Neuroprotection and Induces an Anti-Inflammatory Phenotype in SOD1(G93A) Mouse Model of Amyotrophic Lateral Sclerosis.
Apolloni S, Fabbrizio P, Parisi C, Amadio S, Volonté C. Apolloni S, et al. Mol Neurobiol. 2016 Jan;53(1):518-531. doi: 10.1007/s12035-014-9019-8. Epub 2014 Dec 9. Mol Neurobiol. 2016. PMID: 25482048 - Microglia Polarization From M1 to M2 in Neurodegenerative Diseases.
Guo S, Wang H, Yin Y. Guo S, et al. Front Aging Neurosci. 2022 Feb 16;14:815347. doi: 10.3389/fnagi.2022.815347. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35250543 Free PMC article. Review. - System xC- is a mediator of microglial function and its deletion slows symptoms in amyotrophic lateral sclerosis mice.
Mesci P, Zaïdi S, Lobsiger CS, Millecamps S, Escartin C, Seilhean D, Sato H, Mallat M, Boillée S. Mesci P, et al. Brain. 2015 Jan;138(Pt 1):53-68. doi: 10.1093/brain/awu312. Epub 2014 Nov 10. Brain. 2015. PMID: 25384799 Free PMC article. - The Role of the Innate Immune System in ALS.
Phani S, Re DB, Przedborski S. Phani S, et al. Front Pharmacol. 2012 Aug 14;3:150. doi: 10.3389/fphar.2012.00150. eCollection 2012. Front Pharmacol. 2012. PMID: 22912616 Free PMC article. - Natural products as a potential modulator of microglial polarization in neurodegenerative diseases.
Jin X, Liu MY, Zhang DF, Zhong X, Du K, Qian P, Gao H, Wei MJ. Jin X, et al. Pharmacol Res. 2019 Jul;145:104253. doi: 10.1016/j.phrs.2019.104253. Epub 2019 May 4. Pharmacol Res. 2019. PMID: 31059788 Review.
Cited by
- Modification of Glial Cell Activation through Dendritic Cell Vaccination: Promises for Treatment of Neurodegenerative Diseases.
Sabahi M, Joshaghanian A, Dolatshahi M, Jabbari P, Rahmani F, Rezaei N. Sabahi M, et al. J Mol Neurosci. 2021 Jul;71(7):1410-1424. doi: 10.1007/s12031-021-01818-6. Epub 2021 Mar 13. J Mol Neurosci. 2021. PMID: 33713321 Review. - Capturing Amyloid-β Oligomers by Stirring with Microscaled Iron Oxide Stir Bars into Magnetic Plaques to Reduce Cytotoxicity toward Neuronal Cells.
Tsai YC, Luo JC, Liu TI, Lu IL, Shen MY, Chuang CY, Chern CS, Chiu HC. Tsai YC, et al. Nanomaterials (Basel). 2020 Jun 30;10(7):1284. doi: 10.3390/nano10071284. Nanomaterials (Basel). 2020. PMID: 32629933 Free PMC article. - Microbiome-microglia connections via the gut-brain axis.
Abdel-Haq R, Schlachetzki JCM, Glass CK, Mazmanian SK. Abdel-Haq R, et al. J Exp Med. 2019 Jan 7;216(1):41-59. doi: 10.1084/jem.20180794. Epub 2018 Nov 1. J Exp Med. 2019. PMID: 30385457 Free PMC article. Review. - Transplantation of M2-Deviated Microglia Promotes Recovery of Motor Function after Spinal Cord Injury in Mice.
Kobashi S, Terashima T, Katagi M, Nakae Y, Okano J, Suzuki Y, Urushitani M, Kojima H. Kobashi S, et al. Mol Ther. 2020 Jan 8;28(1):254-265. doi: 10.1016/j.ymthe.2019.09.004. Epub 2019 Sep 10. Mol Ther. 2020. PMID: 31604678 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous