Bridging the Gap between Gut Microbial Dysbiosis and Cardiovascular Diseases - PubMed (original) (raw)
Review
Bridging the Gap between Gut Microbial Dysbiosis and Cardiovascular Diseases
Kimberley Lau et al. Nutrients. 2017.
Abstract
The human gut is heavily colonized by a community of microbiota, primarily bacteria, that exists in a symbiotic relationship with the host and plays a critical role in maintaining host homeostasis. The consumption of a high-fat (HF) diet has been shown to induce gut dysbiosis and reduce intestinal integrity. Recent studies have revealed that dysbiosis contributes to the progression of cardiovascular diseases (CVDs) by promoting two major CVD risk factors-atherosclerosis and hypertension. Imbalances in host-microbial interaction impair homeostatic mechanisms that regulate health and can activate multiple pathways leading to CVD risk factor progression. Dysbiosis has been implicated in the development of atherosclerosis through metabolism-independent and metabolite-dependent pathways. This review will illustrate how these pathways contribute to the various stages of atherosclerotic plaque progression. In addition, dysbiosis can promote hypertension through vascular fibrosis and an alteration of vascular tone. As CVD is the number one cause of death globally, investigating the gut microbiota as a locus of intervention presents a novel and clinically relevant avenue for future research, with vast therapeutic potential.
Keywords: cardiovascular disease; dysbiosis; gut microbiota; prebiotics; probiotics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
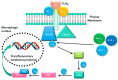
Figure 1
MYD88-mediated TLR4 signaling pathway results in the production of pro-inflammatory cytokines. Activation of TLR4 by LPS through MYD88 dependent pathway results in activation and nuclear translocation of NF-κB which upregulates production of pro-inflammatory cytokines and chemokines. Abbreviations: MYD88 - myeloid differentiation primary response gene 88, TLR4—Toll like receptor 4, LPS—lipopolysaccharides, TIRAP—toll/interleukin-1 receptor domain-containing adaptor protein, MD-2—myeloid differentiation protein-2, CD14—cluster of differentiation 14, IRAK—interleukin-1 receptor-associated kinase, TRAF—tumour necrosis factor receptor associated factor, TAK—transforming growth factor-beta-activated kinase, NF-κB – nuclear factor kappa B.
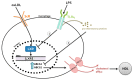
Figure 2
Dysbiosis can induce metabolic endotoxemia, an increased presence of lipopolysaccharides (LPS) in circulation. The LPS-mediated interference of reverse cholesterol transport (RCT) in macrophages occurs (1) directly by reduced expression of LXR and (2) indirectly through mechanisms that upregulate pro-inflammatory cytokines. Abbreviations: oxLDL—oxidized low density lipoprotein, HDL—high density lipoprotein, LXR—liver X receptor, LXRE – Liver X Receptor response element, ScR – scavenger receptor, TLR4 – Toll like receptor 4, ABCA1— adenosine triphosphate (ATP)-binding cassette (ABC) cholesterol transporters including ABC subfamily A member 1.
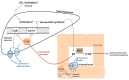
Figure 3
Regulation of cholesterol elimination through bacterial bile-salt hydrolase (BSH) mediated bile acids (BA) activation of hepatic farnesoid X receptor (FXR).
Figure 4
Accumulation of oxidized, low density lipoprotein (oxLDL) in plaque induces vasoconstriction in two ways – upregulation of endothelin-1 expression and inhibition of nitric oxide synthase enzyme. Abbreviations: ETA—endothelin receptor A, ETB—endothelin receptor B.
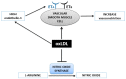
Figure 5
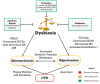
An overview of the relationships between dysbiosis, cardiovascular disease (CVD) risk factors, CVD, and potential treatments. Probiotics, prebiotics and synthetic agents can be used as a treatment for gut dysbiosis which can further prevent the progression of CVDs.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous