Serelaxin improves cardiac and renal function in DOCA-salt hypertensive rats - PubMed (original) (raw)
Serelaxin improves cardiac and renal function in DOCA-salt hypertensive rats
Dong Wang et al. Sci Rep. 2017.
Abstract
Serelaxin, a recombinant form of the naturally occurring peptide hormone relaxin-2, is a pleiotropic vasodilating hormone that has been studied in patients with acute heart failure. In this study, the effects of serelaxin on cardiac and renal function, fibrosis, inflammation and lipid accumulation were studied in DOCA-salt treated rats. Uninephrectomized rats were assigned to two groups: controls provided with normal drinking water and DOCA provided with DOCA pellets and sodium chloride drinking water. After 4 weeks, the DOCA-salt rats were randomly selected and implanted with osmotic minipumps delivering vehicle or serelaxin for another 4 weeks. Treatment with serelaxin prevented cardiac and renal dysfunction in DOCA-salt rats. Serelaxin prevented cardiac and renal fibrosis, as determined by Picrosirius Red staining and Second Harmonic Generation (SHG) Microscopy. Treatment of DOCA-salt rats with serelaxin decreased renal inflammation, including the expression of TGF-β, NFκB, MCP-1, IL-1, IL-6, ICAM-1, VCAM-1 and CD68 macrophages. Serelaxin also decreased lipid accumulation in kidney in part by decreasing SREBP-1c, SREBP-2, ChREBP, FATP1, HMGCoAR, and LDL receptor, and increasing Acox1 and ABCA1. In summary, serelaxin reversed DOCA-salt induced cardiac and renal dysfunction.
Conflict of interest statement
This study was supported by IIS grant support from Novartis to University of Colorado and Moshe Levi. No other conflicts of interest, financial or otherwise, are declared by the author(s).
Figures
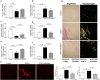
Figure 1
Serelaxin improved heart function of DOCA-salt rats. (a–f) Markers of cardiac hypertrophy and dysfunction as determined by quantitative real-time PCR (qRT-PCR) analysis of α-MHC, β-MHC, BNP, ANF, SERCA in the heart of DOCA-salt rats. Treatment with serelaxin increased α-MHC/β-MHC, decreased BNP and ANF, and increased SERCA. (g) Cardiac fibrosis determined by Picro Sirius Red staining for fibrillary collagens. DOCA induced cardiac fibrosis and treatment with serelaxin prevented the cardiac fibrosis. (h) Cardiac fibrosis as determined by Second Harmonic Generation (SHG) Microscopy showed DOCA induced a marked increase in cardiac fibrosis and that treatment with serelaxin markedly decreased the cardiac fibrosis. Results are expressed as means ± SEM (n = 6 rats). Statistical analysis was performed with one-way ANOVA.
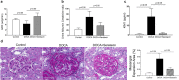
Figure 2
Serelaxin improved renal function of DOCA-salt rats. (a) DOCA induced a decrease in glomerular filtration rate and treatment with serelaxin reversed the decrease in glomerular filtration rate. (b) DOCA markedly increased urine albumin/creatinine excretion rate, and treatment with serelaxin significantly decreased urine albumin/creatinine excretion rate. (c) DOCA induced a marked increase in urinary KIM1 excretion and treatment with serelaxin significantly decreased and normalized KIM1 excretion. (d) DOCA increased glomerular extracellular matrix accumulation and mesangial expansion, as determined by Periodic acid–Schiff (PAS) staining, and treatment with serelaxin markedly decreased the matrix accumulation and mesangial expansion. Results are expressed as means ± SEM (n = 6 rats). Statistical analysis was performed with one-way ANOVA.
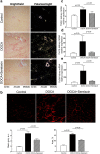
Figure 3
Serelaxin decreased kidney fibrosis of DOCA-salt rats. (a) DOCA increased periglomerular and tubulointerstitial fibrosis, as determined by Picrosirius Red staining, and treatment with serelaxin markedly decreased the periglomerular and tubulointerstitial fibrosis. (b) Second Harmonic Generation (SHG) Microscopy showed DOCA induced a marked increase in tubulointerstitial fibrosis and treatment with serelaxin markedly decreased the fibrosis. Quantitative real-time PCR (qRT-PCR) analysis showed treatment with serelaxin prevented the increases in the (c) profibrotic factor TGF-β, (d) collagen1A and (e) collagen 3 A gene expression in the kidney. Results are expressed as means ± SEM (n = 6 rats). Statistical analysis was performed with one-way ANOVA.
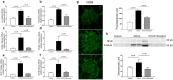
Figure 4
Serelaxin decreased kidney inflammation. Quantitative real-time PCR (qRT-PCR) analysis showed that treatment with serelaxin prevented the increases in the inflammatory markers (a) IL-1-β, (b) IL-6, (c) ICAM-1, (d) VCAM-1, (e) MCP-1, and (f) NFκB mRNA. Serelaxin also prevented the increases in (g) CD68 immunostaining and (h) phospho-NFκB protein abundance in the kidney. Equal protein loading was verified by using an anti-β-actin antibody. The detection was executed by an ECL western blotting analysis system. Whole bolts are reported in Supplemental Fig. 1. Results are expressed as means ± SEM (n = 6 rats for qRT-PCR, n = 3 for western blot). Statistical analysis was performed with one-way ANOVA.
Figure 5
Serelaxin decreased kidney lipid accumulation. (a) Representative images of oil red O staining (20 X), which staining for neutral lipids, showed that increased neutral lipid accumulation in kidney of DOCA-salt rats was prevented by the treatment with serelaxin. The expression of fatty acid, triglyceride and cholesterol metabolism related genes were determined by quantitative real-time PCR (qRT-PCR) analysis, (b) SREBP-1c, (c) ChREBP, (d) FATP1, (e) Acox1, (f) SREBP-2, (g) HMGCR, (h) LDLR, (i) ABCA1. (j) Serelaxin regulated the genes involved in fatty acid, triglyceride, and cholesterol metabolism in DOCA-salt rats. Results are expressed as means ± SEM. Statistical analysis was performed with one-way ANOVA.
Similar articles
- Combining mesenchymal stem cells with serelaxin provides enhanced renoprotection against 1K/DOCA/salt-induced hypertension.
Li Y, Shen M, Ferens D, Broughton BRS, Murthi P, Saini S, Widdop RE, Ricardo SD, Pinar AA, Samuel CS. Li Y, et al. Br J Pharmacol. 2021 Mar;178(5):1164-1181. doi: 10.1111/bph.15361. Br J Pharmacol. 2021. PMID: 33450051 - Comparing the renoprotective effects of BM-MSCs versus BM-MSC-exosomes, when combined with an anti-fibrotic drug, in hypertensive mice.
Li Y, Chakraborty A, Broughton BRS, Ferens D, Widdop RE, Ricardo SD, Samuel CS. Li Y, et al. Biomed Pharmacother. 2021 Dec;144:112256. doi: 10.1016/j.biopha.2021.112256. Epub 2021 Oct 1. Biomed Pharmacother. 2021. PMID: 34607108 - Reversal of cardiac, vascular, and renal dysfunction by non-quinazoline α1-adrenolytics in DOCA-salt hypertensive rats: a comparison with prazosin, a quinazoline-based α1-adrenoceptor antagonist.
Kubacka M, Zadrożna M, Nowak B, Kotańska M, Filipek B, Waszkielewicz AM, Marona H, Mogilski S. Kubacka M, et al. Hypertens Res. 2019 Aug;42(8):1125-1141. doi: 10.1038/s41440-019-0239-1. Epub 2019 Mar 12. Hypertens Res. 2019. PMID: 30872813 - Does the kidney influence the hunger for salt?
Bailey MA. Bailey MA. Kidney Int. 2024 Dec;106(6):1025-1028. doi: 10.1016/j.kint.2024.09.009. Kidney Int. 2024. PMID: 39577987 Review. - Serelaxin: a novel therapy for acute heart failure with a range of hemodynamic and non-hemodynamic actions.
Díez J. Díez J. Am J Cardiovasc Drugs. 2014 Aug;14(4):275-85. doi: 10.1007/s40256-014-0069-0. Am J Cardiovasc Drugs. 2014. PMID: 24590581 Free PMC article. Review.
Cited by
- High-Fructose Diet Increases Renal _ChREBP_β Expression, Leading to Intrarenal Fat Accumulation in a Rat Model with Metabolic Syndrome.
Bier A, Shapira E, Khasbab R, Sharabi Y, Grossman E, Leibowitz A. Bier A, et al. Biology (Basel). 2022 Apr 18;11(4):618. doi: 10.3390/biology11040618. Biology (Basel). 2022. PMID: 35453816 Free PMC article. - Human recombinant relaxin-2 does not attenuate hypertension or renal injury but exacerbates vascular dysfunction in a female mouse model of SLE.
Wolf VL, Phillips TL, Taylor EB, Sasser JM, Ryan MJ. Wolf VL, et al. Am J Physiol Heart Circ Physiol. 2019 Aug 1;317(2):H234-H242. doi: 10.1152/ajpheart.00174.2019. Epub 2019 May 24. Am J Physiol Heart Circ Physiol. 2019. PMID: 31125285 Free PMC article. - HDAC Inhibition Reverses Preexisting Diastolic Dysfunction and Blocks Covert Extracellular Matrix Remodeling.
Travers JG, Wennersten SA, Peña B, Bagchi RA, Smith HE, Hirsch RA, Vanderlinden LA, Lin YH, Dobrinskikh E, Demos-Davies KM, Cavasin MA, Mestroni L, Steinkühler C, Lin CY, Houser SR, Woulfe KC, Lam MPY, McKinsey TA. Travers JG, et al. Circulation. 2021 May 11;143(19):1874-1890. doi: 10.1161/CIRCULATIONAHA.120.046462. Epub 2021 Mar 8. Circulation. 2021. PMID: 33682427 Free PMC article. - ELABELA attenuates deoxycorticosterone acetate/salt-induced hypertension and renal injury by inhibition of NADPH oxidase/ROS/NLRP3 inflammasome pathway.
Chen Z, Wu C, Liu Y, Li H, Zhu Y, Huang C, Lin H, Qiao Q, Huang M, Zhu Q, Wang L. Chen Z, et al. Cell Death Dis. 2020 Aug 22;11(8):698. doi: 10.1038/s41419-020-02912-0. Cell Death Dis. 2020. PMID: 32829380 Free PMC article. - Relaxin-2 as a Potential Biomarker in Cardiovascular Diseases.
Aragón-Herrera A, Feijóo-Bandín S, Anido-Varela L, Moraña-Fernández S, Roselló-Lletí E, Portolés M, Tarazón E, Gualillo O, González-Juanatey JR, Lago F. Aragón-Herrera A, et al. J Pers Med. 2022 Jun 21;12(7):1021. doi: 10.3390/jpm12071021. J Pers Med. 2022. PMID: 35887517 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous