Clinical grade manufacturing of genetically modified, CAR-expressing NK-92 cells for the treatment of ErbB2-positive malignancies - PubMed (original) (raw)
Clinical grade manufacturing of genetically modified, CAR-expressing NK-92 cells for the treatment of ErbB2-positive malignancies
Paulina Nowakowska et al. Cancer Immunol Immunother. 2018 Jan.
Abstract
Background: The NK-92/5.28.z cell line (also referred to as HER2.taNK) represents a stable, lentiviral-transduced clone of ErbB2 (HER2)-specific, second-generation CAR-expressing derivative of clinically applicable NK-92 cells. This study addresses manufacturing-related issues and aimed to develop a GMP-compliant protocol for the generation of NK-92/5.28.z therapeutic doses starting from a well-characterized GMP-compliant master cell bank.
Materials and methods: Commercially available GMP-grade culture media and supplements (fresh frozen plasma, platelet lysate) were evaluated for their ability to support expansion of NK-92/5.28.z. Irradiation sensitivity and cytokine release were also investigated.
Results: NK-92/5.28.z cells can be grown to clinically applicable cell doses of 5 × 108 cells/L in a 5-day batch culture without loss of viability and potency. X-Vivo 10 containing recombinant transferrin supplemented with 5% FFP and 500 IU/mL IL-2 in VueLife 750-C1 bags showed the best results. Platelet lysate was less suited to support NK-92/5.28.z proliferation. Irradiation with 10 Gy completely abrogated NK-92/5.28.z proliferation and preserved viability and potency for at least 24 h. NK-92/5.28.z showed higher baseline cytokine release compared to NK-92, which was significantly increased upon encountering ErbB2(+) targets [GZMB (twofold), IFN-γ (fourfold), IL-8 (24-fold) and IL-10 (fivefold)]. IL-6 was not released by NK cells, but was observed in some stimulated targets. Irradiation resulted in upregulation of IL-8 and downregulation of sFasL, while other cytokines were not impacted.
Conclusion: Our concept suggests NK-92/5.28.z maintenance culture from which therapeutic doses up to 5 × 109 cells can be expanded in 10 L within 5 days. This established process is feasible to analyze NK-92/5.28.z in phase I/II trials.
Keywords: CAR; Cancer immunotherapy; Glioblastoma; HER2; NK-92; Natural killer cells.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
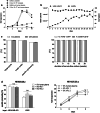
Fig. 1
Impact of culture medium supplements on proliferation and functionality of NK-92/5.28.z. a Cells were seeded in either X-Vivo 10 rTF or CellGro medium, both supplemented with 500 U/mL of IL-2 in the absence (w/o human plasma) or presence of 5% HI human plasma (complete). b Cells were seeded at an initial concentration of 5 × 104/mL in X-Vivo 10 rTF supplemented with 500 U/mL of IL-2 without addition of plasma/serum. Every second day, half the culture supernatant was removed and culture was replenished with fresh, plasma-free medium. Subsequently, cells were re-suspended and cell number and viability were determined. Cells were kept in batch culture for 32 days until reaching confluency defined as 1 × 106/mL. c At the end of the batch culture, identity (CD56+ CD16−), CAR expression (7AAD−CAR+) and specific cytotoxicity against ErbB2(+) targets (MDA-MB-453) were tested and compared with the results obtained with cells cultured in plasma-supplemented medium (with plasma) (left panel). CAR expression and identity were further tested up to 28 weeks (right panel). d Comparison of functionality (left panel) and proliferation (right panel) of NK-92/5.28.z cells cultured in human plasma or hPL supplemented medium. Experiments were performed in triplicates. Data are presented as Mean ± SEM, *P < 0.05
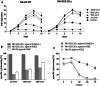
Fig. 2
Impact of IL-2 concentration on proliferation and functionality of NK-92 and NK-92/5.28.z. a Proliferation of parental (NK-92 WT) and genetically modified NK-92 cells (NK-92/5.28.z) in X-Vivo 10 rTF supplemented with 5% of human plasma and various concentrations of IL-2 up to 10 days. b Natural killing of NK-92 (white bars), NK-92/5.28.z (light grey bars) and retargeted killing of NK-92/5.28.z (dark grey bars) cultured for 5 days in X-Vivo 10 rTF supplemented with 5% of human plasma and 50, 100, 500 U/mL of IL-2. c Stability of natural killing of NK-92 (white symbol), NK-92/5.28.z (light grey symbol) and retargeted killing of NK-92/5.28.z (dark grey symbol) in IL-2-free culture was tested up to 72 h
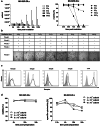
Fig. 3
Radiation sensitivity of NK-92/5.28.z. a Cell concentration (left panel) and viability (right panel) of NK-92/5.28.z cells exposed to **γ-**irradiation were measured every 24 h up to 5 days. b Long-term analysis of NK-92/5.28.z cell proliferation exposed to γ-irradiation. c Receptor expression on irradiated (solid lines) and non-irradiated NK-92/5.28.z cells (dotted lines) 24 h post-exposure. Grey histograms represent isotype matched controls or, in the case of CAR analysis, parental NK-92 cells. d Viability (left panel) and specific cytotoxicity (right panel) of NK-92/5.28.z cells in high-density suspensions were analyzed at the indicated time points over storage under ambient conditions in X-Vivo 10 supplemented with 100 U/mL of IL-2 following γ-irradiation with 10 Gy
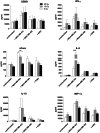
Fig. 4
Influence of irradiation dose on soluble factors released by stimulated NK-92/5.28.z. NK-92/5.28.z were irradiated with 10 Gy (grey bars) and 30 Gy (black bars) and co-cultured with target cells at an E/T ratio of 10:1. NK-92/5.28.z cells cultured in absence of target cells, as well as non-irradiated NK-92/5.28.z (0 Gy, white bars), were included as a control. Concentrations of soluble proteins in test supernatants were measured using a cytometric bead array. Mean values ± SEM are shown; n = 3. **P < 0.01, ***P < 0.001
Fig. 5
Expansion and testing of individual patient dose of NK-92/5.28.z. a According to the established manufacturing process, cryopreserved cells of the ZRX-scFV-anti-ErbB2#78 Master Cell Bank are thawed and continuously cultured for up to 3 months (maintenance culture). Based on an individual prescription of the clinical trial center, NK-92/5.28.z cells are expanded in a VueLife 750-C1 bag until a defined cell density is reached. Cells are harvested, irradiated (10 Gy), washed and transferred in the final bag (CryoMACS 50). Release criteria are checked at this point. NK-92/5.28.z cells are characterized for identity/purity by flow cytometry for the expression of the surface markers CD56+, CD16− and the transgene CAR (≥95%), viability (≥80%), potency (cytolytic activity against an ErbB2(+) target) (≥50%), as well as on the presence of replication-competent lentiviral gene transfer vector. Microbial contamination is analyzed using the BacT/Alert system and the IMP will also be tested for the presence of endotoxins (LAL test) and mycoplasma (PCR). b Three independent batches of NK-92/5.28.z cells were expanded in VueLife 750-C1 culture bags prefilled with 1L of culture medium (left panel). After 5 days, cells were harvested, irradiated with 10 Gy and concentrated to desired density. Final products were tested in terms of viability, CAR expression and specific cytotoxicity (right panel)
Fig. 6
Influence of second-generation ErbB2-CAR expression on the release of soluble factors by stimulated NK-92 cells. NK-92 (white bars) or NK-92/5.28.z (grey bars) at a concentration of 5 × 106 cells/mL were incubated for 2 h with ErbB2(+) MDA-MB-453, ErbB2(−) MDA-MB-468 or K562 cells at an 10:1 E/T ratio. Effector cells incubated without target cells (unstimulated), as well as stimulated with PMA/Ionomycin, served as a negative and positive control, respectively. Concentrations of GZMB, IFN-γ, sFasL, IL-8 and IL-10 were measured in test supernatants. TNF was detected only in the positive control, whereas secretion of IL-2, IL-6, G-CSF and GM-CSF by effector cells was not found (data not shown). Mean values ± SEM are shown; n = 3–4. *P < 0.05, **P < 0.01, ***P < 0.001
References
- Esser R, Muller T, Stefes D, Kloess S, Seidel D, Gillies SD, et al. NK cells engineered to express a GD2 -specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J Cell Mol Med. 2012;16:569–581. doi: 10.1111/j.1582-4934.2011.01343.x. - DOI - PMC - PubMed
- Genssler S, Burger MC, Zhang C, Oelsner S, Mildenberger I, Wagner M, et al. Dual targeting of glioblastoma with chimeric antigen receptor-engineered natural killer cells overcomes heterogeneity of target antigen expression and enhances antitumor activity and survival. Oncoimmunology. 2016;5:e1119354. doi: 10.1080/2162402X.2015.1119354. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 131A009A/German Federal Ministry of Education and Research (BMBF) Cluster für individualisierte Immunintervention, Ci3/International
- 131A009B/German Federal Ministry of Education and Research (BMBF) Cluster für individualisierte Immunintervention, Ci3/International
- 131A009C/German Federal Ministry of Education and Research (BMBF) Cluster für individualisierte Immunintervention, Ci3/International
- III L 5-518/17.004/LOEWE Center for Cell and Gene Therapy Frankfurt (CGT)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous