Understanding the Contribution of Zinc Transporters in the Function of the Early Secretory Pathway - PubMed (original) (raw)
Review
Understanding the Contribution of Zinc Transporters in the Function of the Early Secretory Pathway
Taiho Kambe et al. Int J Mol Sci. 2017.
Abstract
More than one-third of newly synthesized proteins are targeted to the early secretory pathway, which is comprised of the endoplasmic reticulum (ER), Golgi apparatus, and other intermediate compartments. The early secretory pathway plays a key role in controlling the folding, assembly, maturation, modification, trafficking, and degradation of such proteins. A considerable proportion of the secretome requires zinc as an essential factor for its structural and catalytic functions, and recent findings reveal that zinc plays a pivotal role in the function of the early secretory pathway. Hence, a disruption of zinc homeostasis and metabolism involving the early secretory pathway will lead to pathway dysregulation, resulting in various defects, including an exacerbation of homeostatic ER stress. The accumulated evidence indicates that specific members of the family of Zn transporters (ZNTs) and Zrt- and Irt-like proteins (ZIPs), which operate in the early secretory pathway, play indispensable roles in maintaining zinc homeostasis by regulating the influx and efflux of zinc. In this review, the biological functions of these transporters are discussed, focusing on recent aspects of their roles. In particular, we discuss in depth how specific ZNT transporters are employed in the activation of zinc-requiring ectoenzymes. The means by which early secretory pathway functions are controlled by zinc, mediated by specific ZNT and ZIP transporters, are also subjects of this review.
Keywords: ER stress; ZIP/SLC39A; ZNT/Solute carrier family 30 member (SLC30A); early secretory pathway; metallation; tissue non-specific alkaline phosphatase (TNAP); unfolded protein response (UPR); zinc-requiring ectoenzymes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
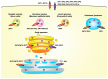
Figure 1
Subcellular localization of ZNT and ZIP transporters. ZNT transporters move cytosolic zinc into the lumen of vesicles involved in the early secretory pathway, including the endoplasmic reticulum (ER), Golgi apparatus, as well as into cytoplasmic vesicles/granules such as synaptic and secretory vesicles and insulin granules, in which specific ZNT proteins are localized. ZNT5 and ZNT6 form heterodimers to transport zinc. ZIP transporters move zinc in the opposite direction. In contrast to the specific localization of ZIP9, ZIP13, and ZIP7 in the Golgi apparatus and the ER, the subcellular location of ZNT5-ZNT6 heterodimers has not been definitively determined.
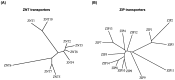
Figure 2
Phylogeny of ZNT and ZIP transporters. The neighbor-joining phylogenetic tree was constructed using ClustalW (
http://clustalw.ddbj.nig.ac.jp/index.php?lang=en
) protein alignment. (A) ZNT and (B) ZIP transporters. Subfamilies and subgroups are designated according to the text.
Figure 3
Model of feedback regulation for the maintenance of zinc homeostasis in the ER (in the early secretory pathway). A disturbance in zinc homeostasis, such as zinc deficiency or zinc overload, in the ER (and perhaps in the early secretory pathway) induces homeostatic ER stress. The unfolded protein response (UPR) leads to the activation of transcription factors such as ATF4, ATF6, and XBP1, and increases the transcription of several ZNT and ZIP transporter genes. These activities of ZNT and ZIP transporters then contribute to the maintenance of zinc homeostasis in the ER (and in the early secretory pathway), and thus attenuate homeostatic ER stress. Zn: zinc.
Figure 4
ZNT5-ZNT6 heterodimers and ZNT7 homodimers function to activate tissue-nonspecific ALP (TNAP) in a two-step mechanism. TNAP is specifically activated in a two-step mechanism involving ZNT5-ZNT6 heterodimers and ZNT7 homodimers as follows: first, the apo-form of TNAP is stabilized by either ZNT5-ZNT6 heterodimers or ZNT7 homodimers; second, the apo-form of TNAP is converted to the _holo_-form by zinc metallation. The PP-motifs in ZNT5 and ZNT7 likely play important roles in this process (see text). TNAP possesses a bimetallic core, is dimeric, and is localized to the plasma membrane via a glycophosphatidylinositol anchor. The subcellular localizations of ZNT5-ZNT6 heterodimers or ZNT7 homodimers have not been well defined. Zn: zinc.
Similar articles
- An overview of a wide range of functions of ZnT and Zip zinc transporters in the secretory pathway.
Kambe T. Kambe T. Biosci Biotechnol Biochem. 2011;75(6):1036-43. doi: 10.1271/bbb.110056. Epub 2011 Jun 13. Biosci Biotechnol Biochem. 2011. PMID: 21670538 Review. - Activation of zinc-requiring ectoenzymes by ZnT transporters during the secretory process: Biochemical and molecular aspects.
Kambe T, Takeda TA, Nishito Y. Kambe T, et al. Arch Biochem Biophys. 2016 Dec 1;611:37-42. doi: 10.1016/j.abb.2016.03.035. Epub 2016 Apr 1. Arch Biochem Biophys. 2016. PMID: 27046342 Review. - Detailed analyses of the crucial functions of Zn transporter proteins in alkaline phosphatase activation.
Suzuki E, Ogawa N, Takeda TA, Nishito Y, Tanaka YK, Fujiwara T, Matsunaga M, Ueda S, Kubo N, Tsuji T, Fukunaka A, Yamazaki T, Taylor KM, Ogra Y, Kambe T. Suzuki E, et al. J Biol Chem. 2020 Apr 24;295(17):5669-5684. doi: 10.1074/jbc.RA120.012610. Epub 2020 Mar 16. J Biol Chem. 2020. PMID: 32179649 Free PMC article. - The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism.
Kambe T, Tsuji T, Hashimoto A, Itsumura N. Kambe T, et al. Physiol Rev. 2015 Jul;95(3):749-84. doi: 10.1152/physrev.00035.2014. Physiol Rev. 2015. PMID: 26084690 Review. - Transcriptome analysis reveals an altered expression profile of zinc transporters in colorectal cancer.
Barresi V, Valenti G, Spampinato G, Musso N, Castorina S, Rizzarelli E, Condorelli DF. Barresi V, et al. J Cell Biochem. 2018 Dec;119(12):9707-9719. doi: 10.1002/jcb.27285. Epub 2018 Aug 20. J Cell Biochem. 2018. PMID: 30129075
Cited by
- Cellular zinc metabolism and zinc signaling: from biological functions to diseases and therapeutic targets.
Chen B, Yu P, Chan WN, Xie F, Zhang Y, Liang L, Leung KT, Lo KW, Yu J, Tse GMK, Kang W, To KF. Chen B, et al. Signal Transduct Target Ther. 2024 Jan 3;9(1):6. doi: 10.1038/s41392-023-01679-y. Signal Transduct Target Ther. 2024. PMID: 38169461 Free PMC article. Review. - Zinc in Prostate Health and Disease: A Mini Review.
Karunasinghe N. Karunasinghe N. Biomedicines. 2022 Dec 10;10(12):3206. doi: 10.3390/biomedicines10123206. Biomedicines. 2022. PMID: 36551962 Free PMC article. Review. - Biogenesis of zinc storage granules in Drosophila melanogaster.
Tejeda-Guzmán C, Rosas-Arellano A, Kroll T, Webb SM, Barajas-Aceves M, Osorio B, Missirlis F. Tejeda-Guzmán C, et al. J Exp Biol. 2018 Mar 19;221(Pt 6):jeb168419. doi: 10.1242/jeb.168419. J Exp Biol. 2018. PMID: 29367274 Free PMC article. - The functional universe of membrane contact sites.
Prinz WA, Toulmay A, Balla T. Prinz WA, et al. Nat Rev Mol Cell Biol. 2020 Jan;21(1):7-24. doi: 10.1038/s41580-019-0180-9. Epub 2019 Nov 15. Nat Rev Mol Cell Biol. 2020. PMID: 31732717 Free PMC article. Review. - Role of Zinc Signaling in the Regulation of Mast Cell-, Basophil-, and T Cell-Mediated Allergic Responses.
Nishida K, Uchida R. Nishida K, et al. J Immunol Res. 2018 Nov 25;2018:5749120. doi: 10.1155/2018/5749120. eCollection 2018. J Immunol Res. 2018. PMID: 30596108 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources