Two novel atypical PKC inhibitors; ACPD and DNDA effectively mitigate cell proliferation and epithelial to mesenchymal transition of metastatic melanoma while inducing apoptosis - PubMed (original) (raw)
Two novel atypical PKC inhibitors; ACPD and DNDA effectively mitigate cell proliferation and epithelial to mesenchymal transition of metastatic melanoma while inducing apoptosis
Wishrawana S Ratnayake et al. Int J Oncol. 2017 Nov.
Abstract
Atypical protein kinase Cs (aPKC) are involved in cell cycle progression, tumorigenesis, cell survival and migration in many cancers. We believe that aPKCs play an important role in cell motility of melanoma by regulating cell signaling pathways and inducing epithelial to mesenchymal transition (EMT). We have investigated the effects of two novel aPKC inhibitors; 2-acetyl-1,3-cyclopentanedione (ACPD) and 3,4-diaminonaphthalene-2,7-disulfonic acid (DNDA) on cell proliferation, apoptosis, migration and invasion of two malignant melanoma cell lines compared to normal melanocytes. Molecular docking data suggested that both inhibitors specifically bind to protein kinase C-zeta (PKC-ζ) and PKC-iota (PKC-ι) and kinase activity assays were carried out to confirm these observations. Both inhibitors decreased the levels of total and phosphorylated PKC-ζ and PKC-ι. Increased levels of E-cadherin, RhoA, PTEN and decreased levels of phosphorylated vimentin, total vimentin, CD44, β-catenin and phosphorylated AKT in inhibitor treated cells. This suggests that inhibition of both PKC-ζ and PKC-ι using ACPD and DNDA downregulates EMT and induces apoptosis in melanoma cells. We also carried out PKC-ι and PKC-ζ directed siRNA treatments to prove the above observations. Immunoprecipitation data suggested an association between PKC-ι and vimentin and PKC-ι siRNA treatments confirmed that PKC-ι activates vimentin by phosphorylation. These results further suggested that PKC-ι is involved in signaling pathways which upregulate EMT and which can be effectively suppressed using ACPD and DNDA. Our results summarize that melanoma cells proliferate via aPKC/AKT/NF-κB mediated pathway while inducing the EMT via PKC-ι/Par6/RhoA pathway. Overall, results show that aPKCs are essential for melanoma progression and metastasis, suggesting that ACPD and DNDA can be effectively used as potential therapeutic drugs for melanoma by inhibiting aPKCs.
Figures
Figure 1
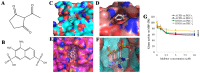
Structures and molecular docking of ACPD and DNDA. Chemical structures of (A) ACPD and (B) DNDA, molecular docking (MD) of ACPD on PKC-ι (C) and PKC-ζ (D) and MD of DNDA on PKC-ι (E) and PKC-ζ (F) are shown. Molecular weights of ACPD and DNDA are 140.14 and 318.32 g/mol, respectively. ACPD interacts with amino acid residues of 469–488 of the catalytic domain of PKC-ι and amino acid residues of 265–290 of the catalytic domain of PKC-ζ. DNDA interacts with amino acid residues of 339–395 of the catalytic domain of PKC-ι and amino acid residues of 337–393 of the catalytic domain of PKC-ζ. (G) Represents the effect of ACPD and DNDA on PKC-ι and PKC-ζ activity. Recombinant active PKC-ζ or PKC-ι were incubated with myelin basic protein in the presence or absence of ACPD and DNDA (0.1–10 _µ_M) and percentage kinase activity was plotted against inhibitor concentration (N=3).
Figure 2
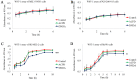
Effects of aPKC inhibitors (ACPD and DNDA) on cell proliferation of normal melanocytes and malignant melanoma cells. Results depict the effect of ACPD and DNDA on PCS-200–013 (A) and on MEL-F-NEO (B), ACPD on SK-MEL-2 (C), DNDA on SK-MEL-2 (D), ACPD on MeWo (E) and DNDA on MeWo (F). Approximately 4×104 were cultured in T25 flasks and treated with either equal volume of sterile water (control) or ACPD or DNDA (0.1–3.5 _µ_M). Additional doses of sterile water or ACPD or DNDA were supplied every 24 h during a 3-day incubation period. Subsequently, cells were lifted and counted. Cell count for PCS-200–013 and MEL-F-NEO cells were only obtained for 3 days due to longer doubling time. The two malignant cell lines (SK-MEL-2 and MeWo) were quantified by counting the viable cells at 24-h intervals. N=3 experiments were performed for each cell line and mean ± SD are plotted. Statistical significance is indicated by asterisks as *P<0.05 and **P<0.01.
Figure 3
Effects of aPKC inhibitors from WST-1 assay for cell viability and cytotoxicity. Cell proliferation was measured using WST-1 assay for (A) MEL-F-NEO, (B) PCS-200–013, (C) SK-MEL-2 and (D) MeWo. The absorbance at 450 nm is due to production of water soluble formazan and was measured as a function of time. The absorbance is directly proportional to the number of cells. Experimental concentrations for both ACPD and DNDA were 2.5 _µ_M and the absorbance at 450 nm against time is plotted. Experiments (N=3) were performed for each cell line and mean ± SD are plotted. *P<0.05 and **P<0.01 indicate statistical significance.
Figure 4
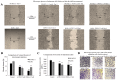
ACPD and DNDA decreases melanoma cell migration and invasion. (A and B) The effect of aPKC inhibitors (2.5 _µ_M of ACPD and DNDA) on melanoma cell migration in wound healing assay and (C and D) represent the effect of inhibitors on melanoma cell invasion in Boyden chamber assay with basement extract. In wound healing assay, microscopic photographs of scratches on cells at the beginning (day 0) were compared with the images taken after 3 or 4 days. The effect of inhibitors are shown (A) compared to their control for both ACPD and DNDA. Experiments (N=3) were performed for each cell line and randomly picked photographs are shown. (B) Represents a comparison of calculated percent wound closure for the photographs taken. For Boyden chamber assay (C), relative fluorescence unit (RFU) values were reported after 2-h exposure of invaded cells with Calcein-AM, as a comparison of control and inhibitors (2.5 _µ_M ACPD and DNDA) treated samples by subtracting the blanks (no cells). (D) A visual representation of crystal violet stained cells which adhered to the bottom of the lower chamber after the invasion. Mean ± SD are plotted in B and C. **P<0.01 indicates statistical significance.
Figure 5
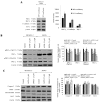
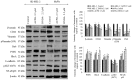
Western blots of aPKC expression in normal melanocytes and the effects of ACPD and DNDA on aPKC expression and apoptosis on malignant melanoma cells. (A) The western blots and densitometry values of expression of PKC-ι, PKC-ζ, E-cadherin and Bcl-2 in MEL-F-NEO normal melanocytes examined at 50 and 100% confluency levels. (B) The expression of phosphorylated PKC-ι, total PKC-ι, phosphorylated PKC-ζ and total PKC-ζ and (C) represents the protein expression of selected apoptotic markers (caspase-3, cleaved PARP, total PARP and Bcl-2) for the ACPD and DNDA (2.5 _µ_M) treated malignant melanoma cell lines (SK-MEL-2 and MeWo) after the end of 3rd day of treatments with respect to their controls. Densitometry bar graphs for (B and C) are shown as the percentage change of the treated samples with respect to their controls and mean ± SD are plotted. A total of 40 _µ_g of protein was loaded into each well and β-actin was used as the loading control in each western blot analysis. Experiments (N=3) were performed.
Figure 6
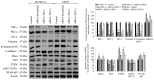
Effect of ACPD and DNDA on EMT signaling pathways. Expression of the protein levels of β-catenin, CD44, vimentin, phosphorylated vimentin, Par6, PTEN, RhoA, E-cadherin, phosphorylated AKT and NF-κB p65 for the ACPD and DNDA treated malignant melanoma cell lines (SK-MEL-2 and MeWo) are shown after the end of 3rd day of treatments with respect to their controls. Densitometry bar graphs are shown as the percentage change of the treated samples with respect to their controls and mean ± SD are plotted. A total of 40 _µ_g of protein was loaded into each well and β-actin was used as the loading control in each western blot analysis. Three experiments were performed.
Figure 7
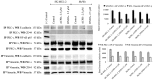
Effect of _si_RNA knockdown of the expression of PKC-ι and PKC-ζ and EMT signaling. Expression of the protein levels of PKC-ι, PKC-ζ, Bcl-2, vimentin, phosphorylated vimentin, Par6, PTEN, RhoA, E-cadherin, phosphorylated AKT and NF-κB p65 for PKC-ι _si_RNA and PKC-ζ _si_RNA treated malignant melanoma cell lines (SK-MEL-2 and MeWo) are shown after the end of 2nd day of treatments with respect to their controls. Densitometry bar graphs are shown as the percentage change of the treated samples with respect to their controls and mean ± SD are plotted. A total of 40 _µ_g of protein was loaded into each well and β-actin was used as the loading control in each western blot analysis. Three experiments were performed.
Figure 8
PKC-ι strongly associates with vimentin. Whole cell lysates (100 _µ_g) of malignant cells (Sk-MeL-2 and MeWo) were IP separately for PKC-ι and vimentin using specific antibodies. First column of the western blot analysis represents the (+) control which contained 40 _µ_g of MeWo whole cell extract, applied to ensure that bands appeared for the specific proteins in western blots. Western blots of PKC-ι IP showed an association with vimentin while no association was observed for E-cadherin, CD44 and NF-κB p65. Vimentin IP confirmed the association with PKC-ι the western blot while no association was observed with above mentioned proteins. Three experiments were performed in each trial. Densitometry for each band is indicated in the bar graph.
Figure 9
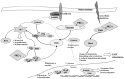
A schematic summary of the involvement of PKC-ι and PKC-ζ in melanoma progression. Upon extra cellular stimulation PKC-ι activate Par6, which leads to the degradation of RhoA and stimulate EMT by changing the cell integrity, loss of E-cadherin and gain of vimentin. During this process, cadherin junctions will be destabilized as a result of loss of E-cadherin and β-catenin will be translocated to nucleus to upregulate the production of some proteins such as CD44 which further stimulate migration and EMT. Importantly, PKC-ι tightly binds to vimentin to activate them by phosphorylation and this activated vimentin changes the cell polarity to maintain the mesenchymal phenotype. Activated vimentin can also stimulate the phosphorylation of PTEN leads to inactivation of inhibitory action of PTEN on PIP3 (48). This may result in activation of AKT through PIP3 and activated AKT pathway leading to cell survival, rapid proliferation and differentiation which are critical parts of melanoma progression. AKT could indirectly stimulate β-catenin translocation and activate NF-κB pathway in which PKC-ζ is known to play a stimulatory role on IKK-α/β. It is reported that activated NF-κB can inhibit PTEN (50).
Similar articles
- Effects of Atypical Protein Kinase C Inhibitor (DNDA) on Lung Cancer Proliferation and Migration by PKC-ι/FAK Ubiquitination Through the Cbl-b Pathway.
BommaReddy RR, Patel R, Smalley T, Acevedo-Duncan M. BommaReddy RR, et al. Onco Targets Ther. 2020 Feb 24;13:1661-1676. doi: 10.2147/OTT.S224866. eCollection 2020. Onco Targets Ther. 2020. PMID: 32158232 Free PMC article. - Oncogenic PKC-ι activates Vimentin during epithelial-mesenchymal transition in melanoma; a study based on PKC-ι and PKC-ζ specific inhibitors.
Ratnayake WS, Apostolatos CA, Apostolatos AH, Schutte RJ, Huynh MA, Ostrov DA, Acevedo-Duncan M. Ratnayake WS, et al. Cell Adh Migr. 2018;12(5):447-463. doi: 10.1080/19336918.2018.1471323. Epub 2018 May 21. Cell Adh Migr. 2018. PMID: 29781749 Free PMC article. - A novel PKC-ι inhibitor abrogates cell proliferation and induces apoptosis in neuroblastoma.
Pillai P, Desai S, Patel R, Sajan M, Farese R, Ostrov D, Acevedo-Duncan M. Pillai P, et al. Int J Biochem Cell Biol. 2011 May;43(5):784-94. doi: 10.1016/j.biocel.2011.02.002. Epub 2011 Feb 16. Int J Biochem Cell Biol. 2011. PMID: 21315177 - Potential therapeutic targets of epithelial-mesenchymal transition in melanoma.
Pearlman RL, Montes de Oca MK, Pal HC, Afaq F. Pearlman RL, et al. Cancer Lett. 2017 Apr 10;391:125-140. doi: 10.1016/j.canlet.2017.01.029. Epub 2017 Jan 25. Cancer Lett. 2017. PMID: 28131904 Free PMC article. Review. - Protein kinase C lambda/iota (PKClambda/iota): a PKC isotype essential for the development of multicellular organisms.
Suzuki A, Akimoto K, Ohno S. Suzuki A, et al. J Biochem. 2003 Jan;133(1):9-16. doi: 10.1093/jb/mvg018. J Biochem. 2003. PMID: 12761193 Review.
Cited by
- aPKC in neuronal differentiation, maturation and function.
Hapak SM, Rothlin CV, Ghosh S. Hapak SM, et al. Neuronal Signal. 2019 Sep;3(3):NS20190019. doi: 10.1042/NS20190019. Epub 2019 Sep 23. Neuronal Signal. 2019. PMID: 32269838 Free PMC article. Review. - Effects of Atypical Protein Kinase C Inhibitor (DNDA) on Lung Cancer Proliferation and Migration by PKC-ι/FAK Ubiquitination Through the Cbl-b Pathway.
BommaReddy RR, Patel R, Smalley T, Acevedo-Duncan M. BommaReddy RR, et al. Onco Targets Ther. 2020 Feb 24;13:1661-1676. doi: 10.2147/OTT.S224866. eCollection 2020. Onco Targets Ther. 2020. PMID: 32158232 Free PMC article. - Juvenile hormone promotes paracellular transport of yolk proteins via remodeling zonula adherens at tricellular junctions in the follicular epithelium.
Zheng H, Wang N, Yun J, Xu H, Yang J, Zhou S. Zheng H, et al. PLoS Genet. 2022 Jun 27;18(6):e1010292. doi: 10.1371/journal.pgen.1010292. eCollection 2022 Jun. PLoS Genet. 2022. PMID: 35759519 Free PMC article. - Targeting Rho GTPase Signaling Networks in Cancer.
Clayton NS, Ridley AJ. Clayton NS, et al. Front Cell Dev Biol. 2020 Apr 3;8:222. doi: 10.3389/fcell.2020.00222. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32309283 Free PMC article. Review. - A Novel Atypical PKC-Iota Inhibitor, Echinochrome A, Enhances Cardiomyocyte Differentiation from Mouse Embryonic Stem Cells.
Kim HK, Cho SW, Heo HJ, Jeong SH, Kim M, Ko KS, Rhee BD, Mishchenko NP, Vasileva EA, Fedoreyev SA, Stonik VA, Han J. Kim HK, et al. Mar Drugs. 2018 Jun 2;16(6):192. doi: 10.3390/md16060192. Mar Drugs. 2018. PMID: 29865255 Free PMC article.
References
- Melanoma of the Skin - Cancer Stat Facts. < http://seer.cancer.gov/statfacts/html/melan.html>, 03/30/2017.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous